
In July 2004, FDA Commissioner Lester M. Crawford, FDA Commissioner announced the desire of the FDA to receive data in a standard format, the CDISC SDTM.

In July 2004, FDA Commissioner Lester M. Crawford, FDA Commissioner announced the desire of the FDA to receive data in a standard format, the CDISC SDTM.

Europe's closest thing to an Oscar's ceremony for bioscience will take place in early October.

In Part 1 of this article, I described a collaboration between Brock Heinz from Spaulding Clinical and Joe Dustin from Medidata, which resulted in a very interesting proof-of-concept.

With all the discussion in Europe of how to move towards personalized medicine and more flexible authorization procedures, it is the rare disease community that is, in many ways, offering guidance on the route ahead.

FDA commissioner Margaret Hamburg has formed a top-level working group to propose strategies for enhancing agency functions and processes, starting with the relationship between FDA Centers and its field force.

In August of 2013, the FDA released a report that analyzed clinical trial subject demographic subgroups for FDA approved medical products, which included factors such as race, age and gender.

As clinical researchers, it?s natural for us to think of patients first and foremost as research participants (potential or current). But that?s not how patients think of themselves.

The Omnibus Final Rule (Final Rule) entitled "Modifications of the HIPAA Privacy, Security, Enforcement, and Breach Notification Rules under the HITECH Act" became effective on March 26, 2013.

The International Academy of Clinical Research (IAoCR) welcomes the news today from the Nursing and Midwifery Council that it is introducing three-yearly checks for nurses and midwifes from the end of 2015 to ensure they are fit to practice.

Industry organizations weigh in on the transparency debate.

A triangle ? an inverted black triangle ? will be seen on the pack leaflets and summary of product characteristics (SPC) of certain medicines in Europe from next week.

With the release of FDA's Guidance on Risk-Based Monitoring (RBM), many clinical operations personnel have been asking how they could implement RBM strategies.

I was lucky to be privy to early results from research being conducted on Risk-Based Monitoring.

New legislation for transparency - currently underway for clinical trial data, national drug pricing rules, and personal privacy - is clearly not enough to satisfy the transparency hawks in Europe.

When we hear about a new blockbuster drug coming to market, we usually think about a medical treatment.

A recent NY Times piece by Tara Parker-Pope reported on the recommendation by a group at the National Cancer Institute to change both the definition of cancer as well as treatment and detection techniques.

JAMA recently announced a change in editorial policy whereby it will no longer have independent academic statisticians review industry-sponsored clinical trial data.

I'm spending some time going through the Draft Guidance for Industry Oversight--A Risk-Based Approach to Monitoring, August 2011 vs. the Guidance for Industry--Oversight of Clinical Investigations--A Risk-Based Approach to Monitoring, August 2013,

In July, at a meeting called by the U.S. Department of Health and Human Services on Human Research Protections, I reviewed the fascinating results of a randomized study that recently compared paper-based informed consent to electronic informed consent.

We?re already embroiled in the annual speculation game about whether FDA approvals this year will keep pace with last year?s near-record of 39 new molecular entities (NMEs) brought to market.

No matter how long you?ve been in the clinical trials business, the mantra is always the same: not enough investigators, not enough patients, enrollment falling behind, need for rescue sites, study budget overspend.

The EMA is committed not just to greater accessibility of data, but proactive publication.

A recent Twitter follower asked in regard to RBM technology, "Is there any experience w/regulators utilizing tool data as a roadmap to ID trouble sites during inspections?"

Accessing and engaging patients is a very difficult task in today’s environment, as there is a bombardment of numerous media vehicles, including digital media, with varying intensities that penetrate patients’ senses, emotions, and thoughts.
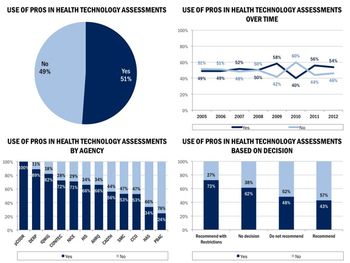
Physicians are increasingly advocating a patient-centered approach to practicing medicine, and both regulatory agencies and manufacturers also seem to agree that patient reported outcomes (PROs) are valuable.

On January 23, 2013, the Department of Health and Humans Services (HHS) published the Omnibus Final Rule (Final Rule) which modifies the "HIPAA Privacy, Security, Enforcement, and Breach Notification Rules under the HITECH Act and the Genetic Information Nondiscrimination Act of 2008."

In the febrile atmosphere of Europe's debates on transparency, could there have been a worse moment for the recent allegations in the UK daily newspaper, The Guardian, that the drug industry is secretly pressuring patient associations to advocate restrictions on the release of clinical trial data?

In the past week, I?ve had two interviews and one webinar all related to some form of safety measuring and diagnosis definition primarily in the CNS area.

A consortium of leading biopharmaceutical companies is moving forward with several projects to make clinical trials more efficient and less costly.
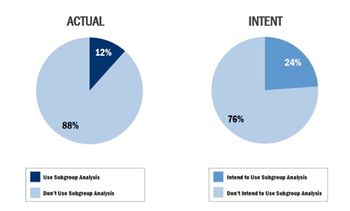
A recent Op-Ed piece in the New York Times by Clifton Leaf asked the question "Do Clinical Trials Work?"