
The EMA's predictions are a measure of optimism against a rather somber background.




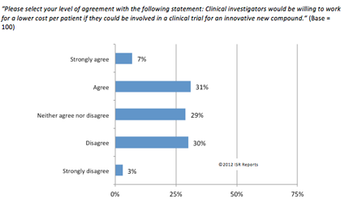

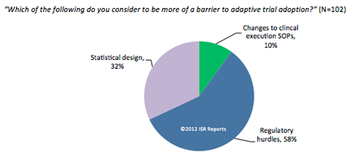

Healthcare is seen as one of the most promising sectors to help boost EU employment.









Will ethics committees buy into the radical new approach of latest draft regulation?
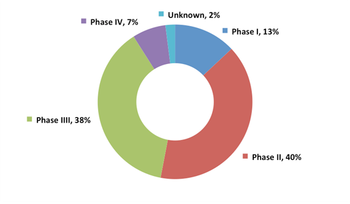





The European Union is preparing a report on the lessons learned from its 2006 pediatric regulation.



