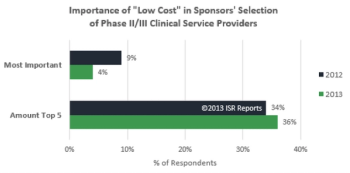
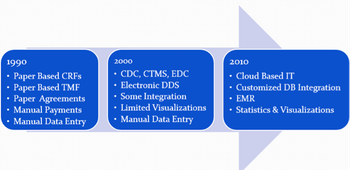
The European Union's bid to update its clinical trials rules, "is particularly important for better competitiveness at global level, by encouraging research and innovation, maintaining a strong pharmaceutical industry and more clinical trials in Europe, and seeking better access to drugs for EU citizens."