
The clinical trial technological landscape continues to morph, as we are starting to see the emergence of novel technologies and techniques to enhance clinical trial strategies and productivity.

The clinical trial technological landscape continues to morph, as we are starting to see the emergence of novel technologies and techniques to enhance clinical trial strategies and productivity.

PCORI is launching new programs that are expected to encourage more analysis of the effectiveness of specific drugs and medical products...

Building an accurate and consistent billing compliance process is a complex task.

The FDA plans to issue numerous draft and final guidances in the coming months...

An insight into how EU membership impacts on medicines and pharmaceuticals has appeared from a British politician

There are a variety of CTMS applications on the marketplace today, and organizations sometimes have a hard time selecting the system that will work the best for them.

The Washington health policy world is headed for a major shake-up next year, as more long-time leading legislators opt for retirement.

In order to manage critical clinical trial information, sponsors and CROs typically implement a strict data management structure...
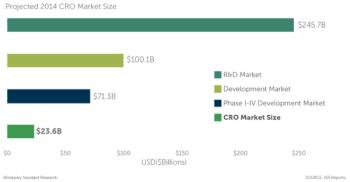
Those of us who have spent considerable time working in the CRO industry have our own version of a "constant" and that is constantly asking the same question: What is the size of the CRO market?

The European Union has a new research program, but will it help, or hinder, clinical trials?

Generating a complete schedule for a hospital department or specialty practice can be a highly complex process.

The drug industry and drug regulatory authorities have been among the most conspicuous targets of this intense monitoring

Collaboration is the way clinical research is moving.

With increasing challenges surrounding clinical trial subject enrollment and engagement, the landscape of clinical trials continues to morph.

Traditionally, clinical trial management systems have been designed as stand-alone applications for either research sites or for organizations

To believe the European Parliament, the battle to create new clinical trials rules for the European Union is over.

After two years in budget limbo, Congress finally enacted federal spending legislation for fiscal year 2014 last week, just before the latest funding extension ran out.

The first conference I?ll attend this year is in two weeks in Philadelphia, on Data Disclosure and Transparency.

Many biopharmaceutical enterprises and CROs are trying to establish solid risk assessment techniques and infrastructures to enhance clinical trial data quality, strategy, and reduce monitoring costs
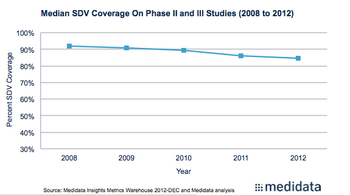
With all of the buzz and excitement around risk-based monitoring, one might expect that the broad adoption of this emerging clinical research paradigm is well under way.

Managing billing compliance is one of the most widely discussed topics in the clinical research community.
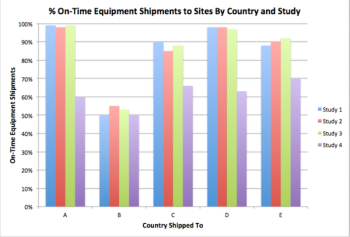
MCC has 100 different metrics relating to clinical trials, from timeliness and cycle time metrics to quality, efficiency, and cost metrics.

As expected, the Food and Drug Administration approved only 27 new molecular entities (NMEs) in 2013.

The U.S. Food and Drug Administration opened the door to a new era of clinical trial management last summer when it released guidance for industry on clinical trial oversight and called for expanded use of risk-based monitoring.

With 2013 having come to a close and gearing up for a what's sure to be a fantastic 2014, it seems an appropriate time to reflect back on some of the most popular content of the year.
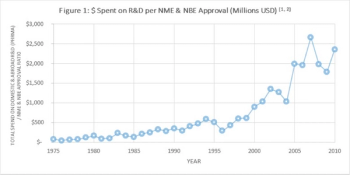
Many of us concur that the biopharmaceutical industry is facing a crisis, and that the R&D process needs to transform entirely in order to address skyrocketing costs and minimal effectiveness.

Drug companies can get more out of their CRO partners by treating them less like avatars and more like equals, according to conference-goers at a CBI event in The Triangle.

Our most read stories of 2013.

It is not only the specific issue of clinical trials rules that hangs in the balance as Europe shifts from 2013 to 2014.

Although a broad compromise was reached in late December on the future shape of the European Union's clinical trials rules, there are still many details to be resolved.