
The Partnerships in Clinical Trials Conference was a large venue that attracted many high-level executives from biopharmaceutical companies

The Partnerships in Clinical Trials Conference was a large venue that attracted many high-level executives from biopharmaceutical companies

The overall burden of drug development has increased exponentially over the last decade or so.

Kent Thoelke spoke at the Partnerships conference regarding trial conduct differences in China.

Five months have flashed past since the CBI's risk-based monitoring event, and so I was looking forward to observing how much the industry has moved on.

A number of top management positions at the Center for Drug Evaluation and Research need to be filled...

The advent of new drugs for tuberculosis is posing some interesting challenges for regulators in Europe.

In January 2013, FDA told manufacturers to lower the dose of zolpidem for women

With the European Union's new rules on clinical trials?including data access?approaching their final legislative stage, and the European Medicines Agency planning to announce its new rules on data disclosure within weeks, the European drug industry is still running to catch up with transparency issues.

I've notice of late quite of lot of confusion over the approval requirements that apply when using mobile devices in clinical trials.

As payers demand more evidence documenting medical product value, biopharma companies are responding by moving sooner to decide key clinical outcomes to measure.

The shift to personalized medicine has been hindered by uncertainty over the value, accuracy, and clinical utility of companion diagnostic tests.

Adaptive licensing has leapt from the pages of learned journals into the real world of European regulation.
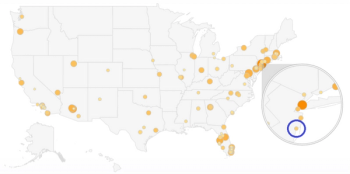
This article will describe my experiences in creating robust strategies for optimizing site selection and offer a few recommendations.

In an effort to reduce costs and improve quality of research, clinical trials are increasingly being offshored.

Biopharmaceutical companies are treading lightly in the use of websites, chat rooms, and interactive online communications to support clinical research programs.

An interview with Dr. Greg Koski about the Alliance for Clinical Research Excellence and Safety (ACRES).
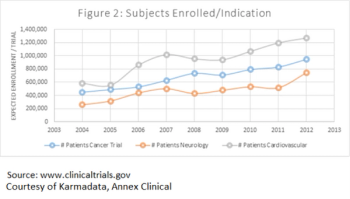
Scientists and experts have conducted extensive research on protocol optimization and the need to enhance study efficiency, and sponsors are starting to look at their study design strategies.

Integrating a CTMS and an EMR can provide many benefits for supporting the health care needs of patients.

For the past few decades, contract research organizations have successfully fulfilled a 'body-shop' role in clinical development...
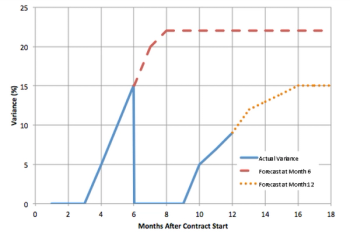
Let's look at a valuable cost metric: percent variance from budget.
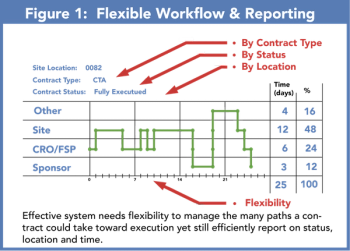
The management and oversight of clinical trials agreement contracts are still handled through spreadsheets or document management systems ill equipped for an efficient CTA lifecycle.

Despite a slight boost in funding for the Food and Drug Administration and stronger tax incentives for investment in R&D, significant changes in Medicare drug reimbursement and coverage policies have biopharmaceutical companies up in arms.

Would you delay protocol approval by two months if it meant your enrollment and retention would improve and, in turn, positively affect your data quality?

The analysis of patterns in clinical trials has indicated that studies are becoming increasingly complex.

Patients communicated alarming behaviors clearly attributed to a lack of communications and engagement from study teams.

In clinical research, it?s essential to know where you're going.

The just-published responses to a UK government evaluation of the merits and demerits of EU membership highlight some of the issues.

Next week Europe will celebrate Rare Disease Day.

In the world of electronic patient reported outcomes, many clinical trial sponsors are interested in the concept of Bring Your Own Device...

Strong working relationships between clinical trials sites and the CRO managing the study are critical to successfully executing a clinical trial.