
A European bid to impose additional limits on research involving human embryos has been defeated.

A European bid to impose additional limits on research involving human embryos has been defeated.

Jean Burns shared experiences with me that offer important insights for government and industry funded clinical research sponsors to consider as they look to improve their partnership with study volunteers.

Effective clinical trial management depends on accurate and unbiased performance measurement.

While adaptive trial designs have been slow to gain traction, there a many benefits to adopting this trial model.

Investments, acquisitions in biotechnology, valuation, and regulatory innovation were main themes at the 2014 NewYorkBIO Conference.

In back-to-back announcements, Boehringer Ingelheim and Bayer HealthCare announced their pathway to allow access of clinical trial data to qualified researchers.

Finding the right CTMS is a long, difficult process.

EMA's crowning achievement in transparency?proactive release of clinical trials data?is only weeks from finalization.

For any Brit involved in medical publishing, The BMJ is the holy grail.

In early May, Bloomberg news reported the FDA will be conducting studies into generic extended release Toprol XL after almost 3,500 adverse incident reports have amassed since March 2009.

This month, let's look at a quality metric that's useful for tracking both protocol and site performance: subject retention percentage.

Amidst all the talk about reducing the time and scope of clinical trials to accelerate drug testing and approval, medical product development still requires researchers and regulators to "get the right answer".
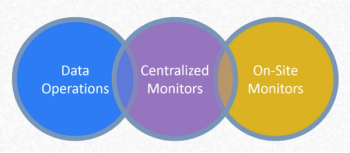
Thomas Verish, Group Director of Data Operations Services at Bristol-Myers Squibb, elaborated on their risk-based monitoring pilot.

In my previous blog "From Science to Fiction" (ACTO, April 15, 2014), I discussed the trials and tribulations of unlearning the scientific writing style...

What is quality? For Patricia Leuchten, CEO of The Avoca Group, and the members of its Quality Consortium, quality can be a platform for innovation and change.

CTMS applications have many features, but for your organization, different features will hold different levels importance.
As I was trying out a series of corrective lenses at an optometrist last week to choose the one that finally provided me the clarity that I was looking for, it made me pose a profound question to myself about perception and reality.

While at the 2014 Bio IT World conference, I had the opportunity to interview Pek Lum, Ayasdi's Chief Data Scientist.

There is plenty of evidence and research which suggests that pharmacies are an excellent medium to engage clinical trial subjects.

The Pfizer-AstraZeneca courtship raises many fascinating questions about the future shape of the international pharmaceutical industry, but it is also revealing some remarkable displays of parochialism.

This year the AACR conference was focused on "Harnessing Breakthroughs, Targeting Cures."

With all of the activity in CRO M&As the past three years, it?s slightly amazing that it continues...
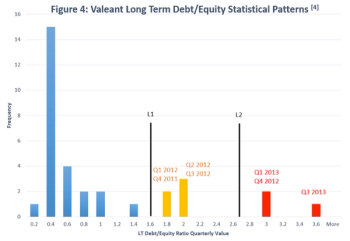
Douglas Tsao, Vice President at Barclays, spoke on the outcomes of Merges & Acquisitions, and its impact on company stock prices.

MCC has just published an executive summary of its Risk Based Monitoring Usage Survey
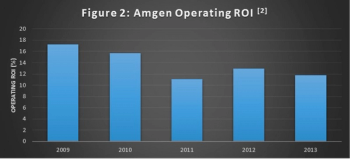
While at the 2014 Partnerships in Clinical Trials Conference, Adrian Otte, VP of Global Development Operations at Amgen, spoke about how Amgen's Risk-Based Monitoring and FSP models impacted business outcomes.
An industry and academic data-sharing project went live on Tuesday, nearly a year after the platform was expected to launch.

Lisa Henderson recaps Roni Zeiger's keynote from the Partnerships in Clinical Trials conference earlier in April.

Almost all of us have said over the years, "With the things I?ve seen, I should write a book."

The European Medicines Agency is determined to minimize opposition to its next moves on releasing clinical trial data.

While the biopharmaceutical industry faces challenges in regulation, standardization, and change, CROs are often in the middle of the change.