
Acknowledging the need to optimize decentralized adoption of innovation in clinical research.
Executive Director and Professor, Tufts University School of Medicine

Acknowledging the need to optimize decentralized adoption of innovation in clinical research.

A new global Tufts CSDD survey of 387 investigative site professionals reveals broad experience with digital and decentralized trial tools, growing site-driven technology investments, and strong support for remote data collection—while highlighting persistent burdens tied to fragmented systems, training demands, and financial strain.

An analysis of primary Phase II protocols paired with their Phase III pivotal trials spotlights the need to balance scientific curiosity with participant and site burden.

Recent analysis explores AI's momentum in drug development, showcasing rapid adoption and significant time savings, despite challenges in trust and data governance.

Anticipating divestment, reallocation of investments, and retrenchment.

Progress made in measuring decentralized clinical trial use in patient recruitment and retention.

Insights identify opportunities to optimize sponsor, CRO, and site collaborations.

Recent research from the Tufts CSDD gauged progress in the context of the “Chief Diversity Officer” role at top pharmaceutical companies.

Collective investment into leveraging popular culture and mass media as an educational medium is urgently needed.

Gathering hard evidence on the benefits and risks of DCT solutions.

New research updates estimated value of lost time.

Spotlight on poor compliance, access, and usefulness of trial results summaries.

Study findings elevate the need to optimize protocol amendment experience.

Amid CVS’ surprise exit from the clinical research space and the ensuing broader questions, study investigates the benefits of pharmacy chain involvement—along with the concerns and barriers to adoption.
Pilot study seeks to validate new and more granular outsourcing model classifications in differentiating performance across custom contract-service approaches.

Investigating more objectively measured 'structural' characteristics.

Tufts CSDD survey finds fostering healthy relationships with site staff is key to a positive outlook.

Self-determined eligibility and the influence of clinical care challenge this flawed figure.

Phesi and Tufts CSDD share results from research on operating practices that can be improved.

Differentiating FSO and FSP approaches can better align definitions of models for CROs and sponsors.

The struggle to translate promising movements in R&D.

Looking beyond regulatory compliance.

Tufts CSDD study in collaboration with ten biopharmaceutical organizations and CROs examines sponsor/CRO relationships.

Characterizing the long-term adoption experience of clinical technologies and capabilities—and what senior leaders can do to drive adoption within their walls.

Insights from studies on advisory boards and participation burden.
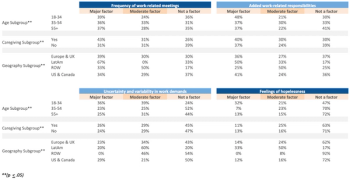
A summary of how organizations approached remote operating models and the experiences and effectiveness of clinical research professionals in their roles and responsibilities during the COVID-19 pandemic.

Affirming and advancing patient engagement and digital transformation.

The drug development sector is embracing technologies and digital methods that were previously not as widely used due to the COVID-19 global health crisis.

2020 Tufts CSDD – IBM Watson Health benchmarking study highlights need for new functionality from EDC solutions and providers.

With an increasing amount of diverse data that must now be collected and analyzed, the industry is faced with increasingly complex studies that present new challenges in data management.

Published: September 8th 2023 | Updated:

Published: March 1st 2013 | Updated:

Published: January 1st 2013 | Updated:

Published: November 1st 2013 | Updated:

Published: May 1st 2013 | Updated:
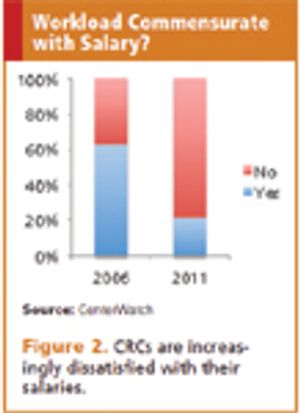
Published: November 1st 2012 | Updated: