
Exploring how large-scale patient databases and AI analytics can accelerate site activation, strengthen recruitment, and improve trial design from the start.
Founder and President, Phesi

Exploring how large-scale patient databases and AI analytics can accelerate site activation, strengthen recruitment, and improve trial design from the start.

Addressing the imbalance in clinical trial workloads by empowering mid-level investigators and using AI to expand access to high-quality, diverse research leadership.

Examining how artificial intelligence can help identify true key opinion leaders in emerging markets to improve site influence, patient engagement, and trial success.

Highlighting how technology and mindset shifts can help expand breast cancer research leadership beyond high-income countries and build more inclusive global trial networks.

Examining how shifting leadership patterns in breast cancer research signal growing international participation—and the continued need for broader equity in global trials.

How targeted AI can improve the performance of clinical trials.

The promise and acceptance of using this AI tool in drug development is growing.

Using existing clinical trial data to proactively model patient safety and efficacy outcomes could aid in accelerating development for areas in need of new therapies.

Already existing enrollment challenges emphasized by single patient sites.

Phesi and Tufts CSDD share results from research on operating practices that can be improved.

Digital twins, defined as a typical demographic patient profile in a specific population, have the potential to help clinical trial design.

Analysis of 330,000 clinical trials calls for improved protocol design process.
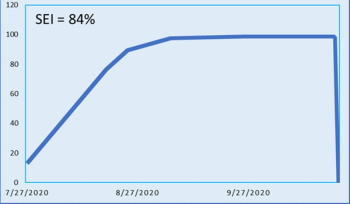
Analysis from Phesi shows the enrollment and effectiveness of two current COVID-19 vaccine trials conducted by global pharmaceutical development companies.
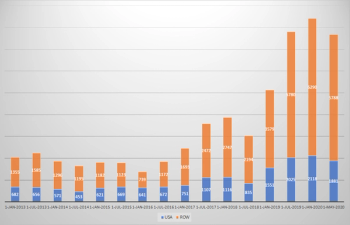
With the COVID-19 pandemic highlighting the existing issues with trial design and execution, the industry needs to address these problems now by becoming data-driven to ensure a long-lasting, positive impact on drug development and commercialization.

Case study shows that site activation is a key driver in determining patient enrollment cycle time, which can be minimized by identifying the "sweet spot" of sites to deploy for a clinical trial.

Case study demonstrates that site activation is a key driver in determining patient enrollment cycle time.
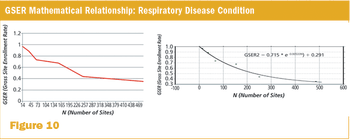
Building a structure for managing study operations through linking trial- and site-level forecasting.

Published: April 13th 2021 | Updated:

Published: April 22nd 2022 | Updated:
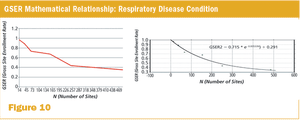
Published: April 1st 2015 | Updated:

Published: January 17th 2018 | Updated:

Published: January 17th 2018 | Updated:
Published: June 9th 2020 | Updated: