Pilot study seeks to validate new and more granular outsourcing model classifications in differentiating performance across custom contract-service approaches.

Pilot study seeks to validate new and more granular outsourcing model classifications in differentiating performance across custom contract-service approaches.

Study by Tufts CSDD uncovers potential reasons behind CRA shortages, turnover, experience requirements, and more.

Tufts CSDD study in collaboration with ten biopharmaceutical organizations and CROs examines sponsor/CRO relationships.

Improving receptivity and response to the evolving nature of clinical trial patient oversight.
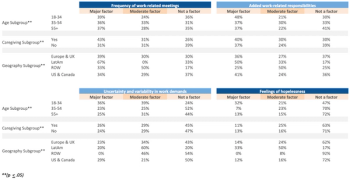
A summary of how organizations approached remote operating models and the experiences and effectiveness of clinical research professionals in their roles and responsibilities during the COVID-19 pandemic.
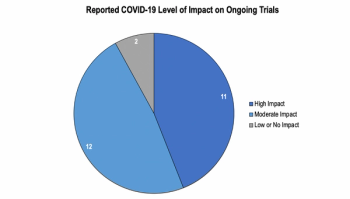
Findings from a Tufts study examining the effects of COVID-19 on clinical trials.

Tufts Center for the Study of Drug Development and Biogen recently conducted a study to inform growing interest in improving diversity of clinical trial participation. The results of this research provide insights into increasing the community of minority investigators and study staff and presenting greater access to clinical trials among minority study volunteers.
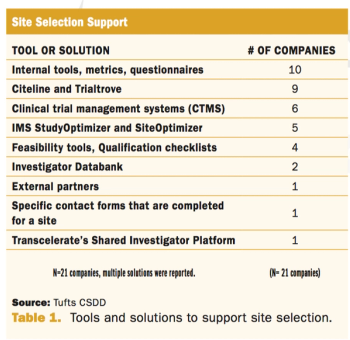
Assessing practices and inefficiencies with site selection, study start-up, and site activation.

Study collects first comprehensive metrics on current supply management and distribution practices.
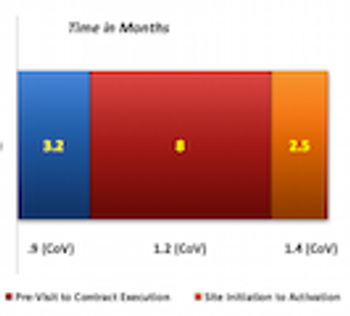
Study finds that despite the use of new approaches to streamline and accelerate study start and improve site selection and activation, the impact on start-up cycle times has been limited. The reasons why are explored.
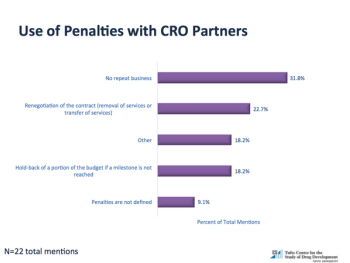
Demand for outsourced services to provide clinical development capacity and expertise has grown substantially over the last 10 years.

Pressure to shorten study start-up timelines puts clinical supply management in the crosshairs.

Sponsor companies and service providers have embraced more strategic management of their clinical supplies while regulatory pressures and the volume of global clinical trial activity have increased.

From tactical to strategic: tracking the evolution of global clinical supply chain management.
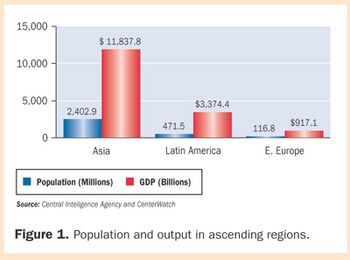
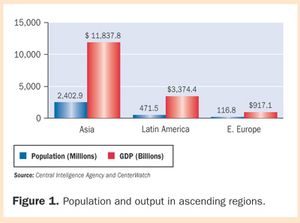
Clinical trials in developing nations-called "ascending markets"-are exploding. These regions offer large naïve subject populations, low operating costs, and increasingly stable testing infrastructures, but regulatory reform is really driving the growth. CenterWatch data show 20%?30% of clinical trials are being conducted in ascending regions.

Published: November 15th 2021 | Updated:

Published: September 1st 2012 | Updated:
Published: June 1st 2004 | Updated:

Published: May 12th 2014 | Updated:

Published: August 1st 2014 | Updated:
Published: August 11th 2015 | Updated: