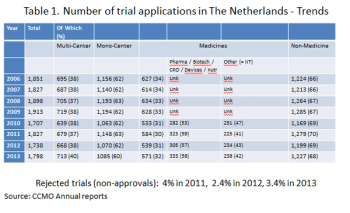
Over the past few years, the number of active, accredited Ethics Committees (EC) in the Netherlands has dropped at a steady pace. The decline over the past year has partly been due to the merger of several ECs.

Over the past few years, the number of active, accredited Ethics Committees (EC) in the Netherlands has dropped at a steady pace. The decline over the past year has partly been due to the merger of several ECs.


The European Medicines Agency (EMA) has launched the public consultation on how the transparency rules of the European clinical trial regulation will be applied in the new database.

To improve visibility, control, and collaboration for its ongoing and planned clinical trials, San Diego-based biotech Conatus Pharmaceuticals selected Veeva Vault eTMF and Veeva Vault QualityDocs – cloud applications for the management of clinical study and quality and development documentation, respectively

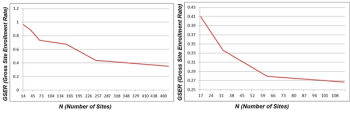
Instinctively, when there are more investigators/sites being deployed for a trial with a defined number of patients needed, we should expect shortened enrollment cycle time. This sounds right, but is it really?

Efforts are escalating to encourage sponsors, research institutions and clinical investigators to accept oversight for multi-center studies by central Institutional Review Boards (IRBs).

The need for innovation in clinical trials has sparked significant interest from a variety of new enterprises including technology, analytics, subject enrollment/engagement/retention, EDC, risk-based monitoring, and many mor

Pharmaceutical Product Development, LLC (PPD) has expanded its good manufacturing practices (GMP) operations in Athlone, Ireland, by adding a new, state-of-the-art laboratory for cell-based assays to its existing portfolio of services at the facility.

Analytical testing facility adds state-of-the-art cell-based lab capabilities

Clinical Conductor, the industry’s most collaborative CTMS, was recently fitted with new features and functionality that provide users with even more capability to connect with other research technologies and further collaborate with research partners to ensure clinical research success.

With the US Food and Drug Administration (FDA) approving an all-time record number of orphan drugs during 2014, the pricing of these treatments is set to come under increased scrutiny.

Research and consulting firm GlobalData says the FDA approved an all-time record number of 17 orphan drugs during 2014

Covance has introduced its Early Phase Development Solutions, a multi-disciplined approach to early drug development.

Covance Inc., the world’s most comprehensive drug development company, today announced the introduction of Covance Early Phase Development Solutions,

eClinical Solutions, LLC, a leading provider of data management services and technologies, today announced the release of elluminate®.

eClinical Solutions, LLC, a leading provider of data management services and technologies, today announced the release of elluminate®.

How the European Union is impacting current Pharmaceutical trends.


Clinovo launches the latest version of its Electronic Data Capture (EDC) system ClinCapture.

Web-based and iPad tablet application solution for writing investigator-initiated research protocols

Clinovo launches the latest version of its Electronic Data Capture (EDC) system ClinCapture.

Europital announces the official launching and certification attainment of its latest product.

Europital announces the official launching and availability of its electronic platform PenThu.

Global clinical trial performance and efficiency are hampered by high turnover and noncompliance among principal investigators and wide variation in investigative site experience.

Global clinical trial performance and efficiency are hampered by high turnover and noncompliance among principal investigators and wide variation in investigative site experience.

Industry news focusing on the people and organizations who work in the clinical trials profession.

Life sciences organizations run a challenging gauntlet when it comes to clinical development.

Fundamental weaknesses of modern clinical development can be resolved through recent statistical advances.

CROMSOURCE is delivering several modules of a training course on clinical research best practice organized by the SIFC (Italian Cystic Fibrosis Society).