
News



Accelovance, Inc. , a CRO therapeutically aligned in the areas of Oncology, Vaccines, and General Medicine; today announced it has acquired Altair Clinical, Ltd.


Astellas Pharma Inc. (Tokyo: 4503, "Astellas") announced today that the company will now make trial data available through www.clinicalstudydatarequest.com,


ICON, a global provider of outsourced development services to the pharmaceutical, biotechnology and medical device industries, today announced it has agreed, subject to certain customary closing conditions, to acquire MediMedia Pharma Solutions for a cash consideration of $120 million.


ERT, a leading provider of technology solutions and services that increase the reliability and efficiency of high-quality patient data collection, today announced that its electronic Suicide Risk Assessment (SRA) system – AVERT® – will be implemented by Rutgers University

The "discussion draft" for legislation to speed "21st Century Cures" to patients emerged very quietly on Capitol Hill recently, muted by an absence of bipartisan support which had generated considerable enthusiasm for this effort to promote biomedical research and streamline regulation.

Efforts are escalating to encourage sponsors, research institutions, and clinical investigators to accept oversight for multi-center studies by central institutional review boards (IRBs), as seen in several discussions of this topic at the December conference on "Advancing Ethical Research" sponsored by Public Responsibility in Medicine & Research.

In early January, Novartis selected Qualcomm Life as a global digital health collaborator for its Trials of The Future program.

The World Health Report 2013 argues that universal health coverage cannot be achieved without the evidence from scientific research, and in order to better manage healthcare, clinical research needs to keep up-to-date and advance in line with society in general.

A surge in review activity at the FDA in December resulted in a near-record approval of 41 new drugs and biologics last year, the most since a record 56 approvals in 1996.

Agency proposes new standards in hopes of capitalizing on 2014 surge in new drug approvals.

The five-year figure doesn't always determine which cancer types are most commonly being studied in late-phase trials.

Sites: Integrated Clinical Research Boosting the Investigator Pool Trial Design: Remote Patient-Centered Studies Risk-Based Monitoring in Action Also in this issue: New Drug Approval Momentum mHealth in Clinical Trials Simplifying Protocol Design
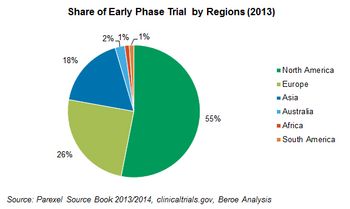
The developed markets of North America and Europe together saw approximately 81% of the Phase I trial share in 2013 which is a 6% increase from 2012. Trials

The Clinical Trials Transformation Initiative (CTTI) has announced recommendations to streamline Good Clinical Practice (GCP) training of investigators who participate in clinical trials.


The idea is great: when an investigator submits a grant to any NIH institute, if the study will involve more that one performance site, the grant must include a plan to rely on one single IRB.

One way to reduce off-label prescribing of treatments for cancer and other conditions is for manufacturers to test additional indications and file supplemental applications to add those uses to approved labeling

TransCelerate BioPharma announced two new members, Merck & Co. Inc., and Novo Nordisk.

TransCelerate BioPharma Inc. today announced two new members, Merck & Co. Inc., and Novo Nordisk, to the biopharmaceutical non-profit organization.

ArisGlobal, a leading provider of solutions to the life sciences industry, has announced that agDisclosure, its clinical trial disclosure solution, now supports Version 10 of the European Medicines Agencys EudraCT database

ArisGlobal, a leading provider of solutions to the life sciences industry, has announced that agDisclosure, its clinical trial disclosure solution, now supports Version 10 of the European Medicines Agencys EudraCT database

Forty-three percent of pharma and life sciences execs now support FDA evaluating drugs based on both clinical and economic effectiveness

Consumers and drug and device manufacturers are changing practices and shifting attitudes toward the Food and Drug Administration (FDA). Increased pressures for speedy access to breakthrough drugs and medical devices, and a focus on value in addition to medical benefit, are driving these changes.

Improves data quality and regulatory compliance by combining scientific training with innovative electronic implementation of instruments

The pharmaceutical industry is further optimizing the collection of high quality electronic patient reported outcomes (ePROs) data for clinical research, with PHT Corporation Rater Training programs