
Certaa announced that its regulatory and medical consultancy, Synchrogenix, has acquired ClinGenuity.

Certaa announced that its regulatory and medical consultancy, Synchrogenix, has acquired ClinGenuity.

Deal provides Synchrogenix withproprietary technology, expert staff, and new capabilities to become the dominant player in life science regulatory and medical writing

Applied Clinical Trials is pleased to announce our sponsorship of Partnerships in Clinical Trials' Partnership Hall of Famer Award, as well as its Clinical Operations Optimization track.

The entry into force of the new EU clinical trials regulation last June is really little more than a virtual event.

Mapi has completed the acquisition of select Optum Life Sciences services.

Mapi Group completes the acquisition of select Life Sciences services.

Françoise Meunier will become Director of Special Projects for the European Organization for Research and Treatment of Cancer starting April 1, 2015.

At the Crowne Plaza Brussels - Le Palace on 12 March 2015 at 16:00

It's official. Europe is sick-and much of the sickness derives from its economic problems.
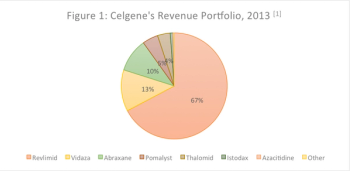
This article will analyze the impact of Revlimid on a different outcome: multiple myeloma mortality rates in the US.

PPD and Shin Nippon Biomedical Laboratories Ltd. announced an agreement to form a joint venture.

PPD and Shin Nippon Biomedical Laboratories Ltd. announced an agreement to form a joint venture.

The Board adopts EMA work program 2015-2016

The Management Board of the European Medicines Agency (EMA) has outlined its priorities for the next two years.

Michael J. Rosenberg died on December 8 in an accident while en route to a meeting at the Food and Drug Administration.
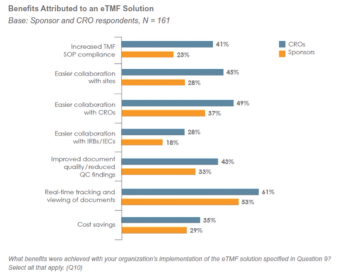
All signs indicate that 2015 should be another good year for contract research organizations (CROs).

Patient access to critical experimental medicines continues to grab public attention, as states enact “Right-to-Try” laws and Congress eyes establishing a national policy to provide not-yet-approved therapies to terminally ill patients.

FDA has scheduled a public meeting in early January to assess and weigh the data on the first US application for a biosimilar therapy.

After nearly five years of argument, the European Union's proposal to update its transparency directive for medicines has been dropped.

As of November 2014, the European Medicines Agency (EMA) had received and assessed 29 applications as part of its pilot project on adaptive pathways, formerly known as adaptive licensing.

Building on more than a decade of initiatives to spur pediatric labeling on drugs and biologics, regulatory authorities are bolstering incentives for sponsors to develop more information faster on the use of medicines in children.

Scientists argue that scientific and political changes will make adaptive pathways the preferred approach to make new treatments available

New Cardiac Safety Methodology from iCardiac Technologies Offers Earlier Cardiac Safety Characterization and Significant Cost Savings

For robust scientific assessment more information on safety and efficacy needed.

While the issue of adverse drug reaction (ADR) reporting is apparent in hospital systems, a deeper and uninvestigated problem resides within the process of ADRs: the patient.

There is not enough available evidence yet for any of the experimental therapies for the Ebola virus disease to draw conclusions on their safety or efficacy when used in Ebola patients, according to an interim report published by the European Medicines Agency.
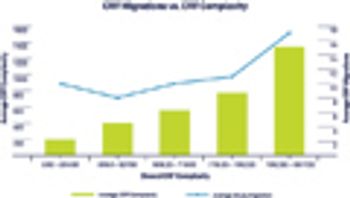
Medidata took a look at how eCRF design complexity correlates with the number of migrations performed while a study is actively collecting subject data.

In the coming year, we expect to see sponsors focused on making better use of their data investments that are now electronically available through EDC and other systems.

Granted Permission to Host its Own Web Version of the EQ-5D

PPD and ERT announced a strategic partnership.