
Clinverse, Inc. and the Alliance for Clinical Research Excellence and Safety announced today that they have formed a strategic partnership.

Clinverse, Inc. and the Alliance for Clinical Research Excellence and Safety announced today that they have formed a strategic partnership.

New PRO measure being developed in conjunction with the FNIH Biomarkers Consortium

Growing insistence on making healthcare more patient-centered is generating a new level of interest in helping patients to meet the expectations the new orthodoxy is creating.

ERT announced the acquisition of eClinical Insights

The 2014 mid-term elections handed over control of the Senate to Republicans and boosted the GOP majority in the House.

Clinverse and ACRES announced a strategic alliance.

Combined Offering Enables Real Time Decision Making in Global Clinical Trials

Here is a summary of what analysts are saying about LabCorp's purchase of Covance.

Drugs that treat central nervous system diseases take more than a year longer to develop and are less than half as likely to obtain marketing approval

Joint Effort to Enable Faster, Safer and Smarter Clinical Trial Reviews for Life Science Industry

EUCROF released proposals on the early publication of clinical trial information, balancing the public's need for transparency and innovators' intellectual property and confidentiality rights.

OPKO Health to build and configure integrated data capture and drug safety systems

Drugs that treat central nervous system diseases take more than a year longer to develop

EUCROF has unveiled proposals on the early publication of clinical trial information.

Medidata and PPD announced they are expanding their partnership around risk-based monitoring.

This position paper has been prepared by the European Contract Research Organisation Federation (EUCROF).

LabCorp and Covance have entered into a definitive agreement
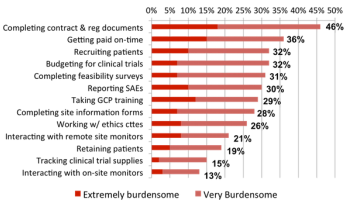
The sharp rise in ongoing clinical research studies is driving demand for greater participation in research by physicians as well as by patients.

So now we know who is to do what on health issues in the new European Commission. Or do we?

Covance shareholders to receive cash and LabCorp shares currently valued at $105.12 per Covance share

We hear it all the time these days: research processes have to undergo transformative changes in order for research organizations to thrive?or even survive.

There's a mounting campaign to encourage biopharmaceutical companies to develop new therapies to treat drug-resistant infections.

Velos recently activated its Investigational Drug System at The University of Texas MD Anderson Cancer Center
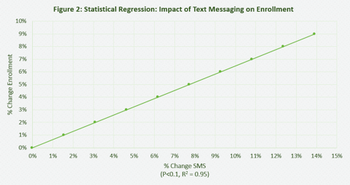
There is a lot of new research that evaluates the impact of Short Messaging System (SMS, Text Messages) in healthcare settings.

Over 100 MD Anderson pharmacists and technicians across 15 dispensing pharmacies began using the Velos Internet-based software

KMR Group has recently assessed the duration of oncology trials.

KMR Group recently assessed the duration of oncology trials and found a steady rise

Central laboratory analysis of samples collected by the investigational sites is an important source of safety and efficacy endpoint data.

Medidata announced that it has signed an enterprise agreement with ASLAN Pharmaceuticals