
Clinical Phase Company Adopts Medidata Platform to Bring Operational Efficiencies and Greater Speed to Its Clinical Trials Across Asia

Clinical Phase Company Adopts Medidata Platform to Bring Operational Efficiencies and Greater Speed to Its Clinical Trials Across Asia

The European Business Association came out in support of clinical trials in Ukraine.

Developers encouraged to request accelerated procedure for scientific advice
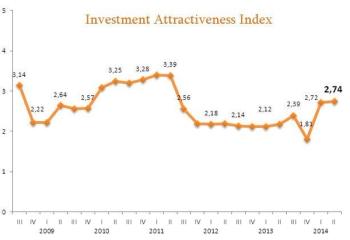
Following our practice of supporting European Business Association member-companies in their business activities in Ukraine, let us kindly inform you on the current state of the clinical trials industry in Ukraine.

European Medicines Agency has given 'rapid scientific advice'

Leading Senators are proposing legislation to add Ebola to the list of diseases eligible for priority review vouchers from the FDA.

The first patient expert training course of the European Patients' Academy (EUPATI) has begun.

EUPATI welcomes 53 participants to educational program on medicines' R&D

Addition of Respected Clinical Trials Company Expands MPI Research Capabilities to Include Human Phase I Testing

MPI Research announced it has acquired the assets Jasper Clinical Research & Development, Inc

The Association of Clinical Research Organizations today announced plans to constitute a CRO Forum

What's been a commonplace technology for larger organizations is now becoming more accessible economically to smaller enterprises. But how to choose?
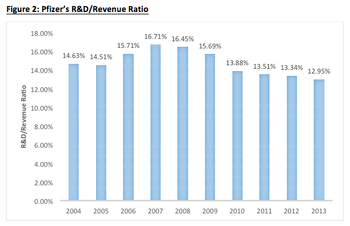
Is Pfizer prime for acquiring another biopharmaceutical company?

Pharmaceutical companies took advantage of East Germany's financial problems, report found.

Drug firms took advantage of East Germany's financial problems to carry out trials with ethically uncertain processes, say researchers

The Chancellor of the Exchequer announced that a partnership led by the Medical Research Council will invest over ?230 million

Issues being discussed with the Ebola vaccine sound like issues discussed with clinical trials in general. What can we learn?

The Clinical Research Infrastructure Initiative will benefit from additional funding from the UK government

inVentiv Health Clinical will replace its eTMF content management system with Veeva Vault eTMF.

Clients benefit from complete access, visibility, and control throughout the clinical trial
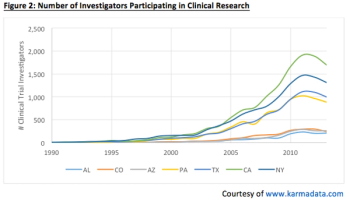
There are a number of indicators that suggest that US clinical trial growth is far from plateauing, and demand for clinical research investigators will continue to rise with this growing trend.

CRO to provide comprehensive clinical research management and support

Spoiler alert: most IRT systems are not meeting user expectations in terms of eClinical integration.

Rho has been awarded the renewal of their existing federal contract with the National Institute of Dental and Craniofacial Research

Almac announces the launch of its "Direct to Patient" shipping service for clinical trials

Available for clinical studies conducted throughout the US & Canada

Radiant Sage, LLC announced the newest version of its Corelab-in-a-Box

ClinicalTrials.gov

New Version of Clinical Trial Imaging Management Solution Expands Features for Specific Clinical Areas, Enhances Workflow, Increases Flexibility, and Eases Quality Control Processes