
One of the primal questions clinical trialists engaged in clinical research and development always face is do we “keep or kill” a drug based on the data in hand.

One of the primal questions clinical trialists engaged in clinical research and development always face is do we “keep or kill” a drug based on the data in hand.

The deal will enable Synexus to further expand both its global network of clinical research centers - into the US, Asia and South America - and the range of therapy areas it focuses on.

The deal will enable Synexus to further expand both its global network of clinical research centers - into the US, Asia and South America - and the range of therapy areas it focuses on.

Global network accelerates patient recruitment and clinical trial start-up times


IntroductionAfter decades of promise, we have begun to realize the fruit of “-omics” technology. Recent advances in proteomics, genomics and metabolomics have enabled us to understand the molecular basis of disease at both the diagnostic and treatment levels. Equally important, a growing suite of biomarkers now provides predictive value for diagnosis, disease progression and cure/remission.



Pearl IRB earned this distinction in December 2014 by demonstrating extensive safeguards in all levels of the research operations and abiding by high standards of excellence for all research.


CRF Health, a global provider of electronic Clinical Outcome Assessment solutions for the life sciences industry, announced a new partnership with Vodafone.


Theorem Clinical Research, a prominent global contract research organization, and Biomedical Systems, a leading provider of comprehensive centralized diagnostic services, have formed a strategic partnership

PHT next week will announce a new suite of clinical research patient engagement apps at the Summit for Clinical Ops Executives conference in Orlando

Patient adherence is an emerging topic and a matter of concern in clinical research. It applies to several different clinical trial facets.


Leading Biopharma Company Adopts Medidata Technology to Bring Operational Efficiencies and Greater Speed to Innovative Clinical Study on Metabolic Disorders
What leads to Patient dropout? Read more here.
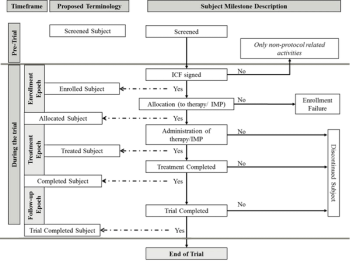
Subject disposition, which is the flow of patients through a clinical trial, requires further discussion for harmonization of terms.

At a time of the year when love is in the air, one thing is for sure: there is not a lot of love from the human research community on NIH’s Policy on the Use of a Single IRB for Multi-Site Research.

PCI is pleased to announce that further to the acquisition in September 2014 of Biotec Services International, the company has rebranded as part of the PCI group.


The ongoing battle over drug reimbursement and pricing has raised questions about whether the pharmaceutical industry can continue to rely on high U.S. revenues to fund biopharmaceutical R&D. Payers and insurers have become more aggressive in demanding clear evidence of value for high-priced medicines and are rejecting old models for coverage and reimbursement.
In early January, Novartis selected Qualcomm Life as a global digital health collaborator for its Trials of The Future program. That program is designed to leverage healthcare technology to improve the experience of clinical trial participants and patients using Novartis products.

Medidata, the leading global provider of cloud-based solutions for clinical research in life sciences, today announced a strategic collaboration with Garmin International Inc.


ClinPlan provides automated, efficient, and accurate way to assess and reassess trial expenses over time throughout the life of the trial.

Clinverse, Inc., provider of automated financial management technology solutions for clinical trials, announced today that it has expanded its suite of products with the addition of ClinPlan™, purpose-built software for clinical trial budget management and forecasting.