
News


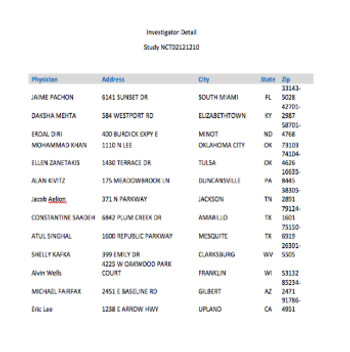
With Open Payments US investigator names and experience will become public knowledge.
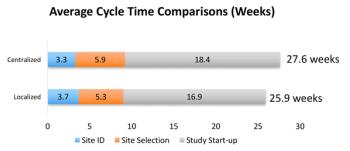
Numerous factors can adversely impact study startup and its efficiency, in an industry plagued by rising development costs and increasing complexities.

Updated employee announcements, business news, awards, and recognition in the industry today.



Outlining the growing attention and pursuits around social media in adverse event reporting and advancing patient-centric initiatives in clinical trials.



FDA funding increased under Trump's budget for 2019 fiscal year while resource reductions take place for NIH.



The 10th anniversary of the Clinical Trials Transformative Initiative provided an opportunity for FDA officials to join with study sponsors and research experts to examine the policy achievements and plans for future efforts of improving clinical trials.

During the CROWN congress, there was discussion on CRO acquisition and consolidation, growing interest in the use of Blockchain technology in clinical trials, and new patient engagement methodologies.



In this interview, Jennifer Prichard, MD, Medical Monitor at Atlantic Research Group, and Hunter Walker, CTO at Atlantic Research Group will discuss challenges with existing medical monitoring processes, and how new technologies can help improve efficiency.





Highlighting three distinct patient partnership models to help researchers evaluate which methods of engagement could work best for their clinical programs.






