
Outlining the unique pharmacokinetic factors that should be considered when designing and running early stage clinical trials for monoclonal antibodies.

Outlining the unique pharmacokinetic factors that should be considered when designing and running early stage clinical trials for monoclonal antibodies.

Sobering statistics collected on clinical trial execution point to the eventual convergence of healthcare and clinical research operating environments.

Click the title above to open the Applied Clinical Trials June 2018 issue in an interactive PDF format.




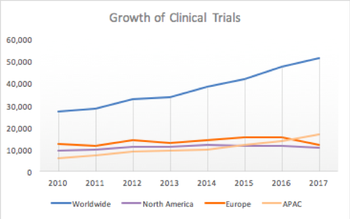
As international outsourcing continues to drive the parties contributing to clinical trials farther apart, technology will increasingly serve as a means of bringing them closer together.



John Reites, THREAD’s Chief Product Officer, will discuss eDROs and the FocalView App in this interview.

Right-to-Try bill sent to White House for President’s signature after passage by Congress.


Artificial intelligence is a "new weapon" in healthcare research.

From 2019, to comply with requirements for greater transparency around clinical research as part of the EU Clinical Trials Regulation 536/2014, life sciences firms will be expected to prepare plain-language summaries for all Phase I through IV interventional trials, which can be understood by patients, the general public, and experts.




When less is more. Using three referral labs vs. one central lab.
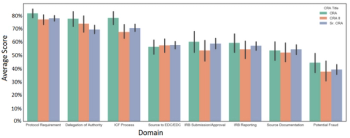
A global CRO’s data gathered using an objective monitoring simulation administered by CRA Assessments, LLC reveals that CRAs are consistently underperforming regardless of the level of experience or training.

With the rise in gene therapies entering clinical trials, it is important to address the operational challenges associated with these types of trials.

The biopharmaceutical industry continues to explore how mHealth can change clinical trials, as the discussion continued at Hanson Wade’s mHealth for Clinical Trials EU Summit in London.





