
News



When less is more. Using three referral labs vs. one central lab.
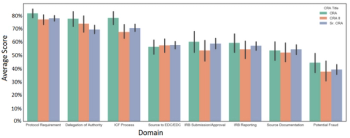
A global CRO’s data gathered using an objective monitoring simulation administered by CRA Assessments, LLC reveals that CRAs are consistently underperforming regardless of the level of experience or training.

With the rise in gene therapies entering clinical trials, it is important to address the operational challenges associated with these types of trials.

The biopharmaceutical industry continues to explore how mHealth can change clinical trials, as the discussion continued at Hanson Wade’s mHealth for Clinical Trials EU Summit in London.






Updated employee announcements, business news, awards, and recognition in the industry today.

On Europe Day, the agency itself is right now keener than ever to assess its worth and the value of its services to Europe and to Europeans.







In this interview, Munther Baara, Head of New Clinical Paradigms at Pfizer, will discuss his perspectives on how blockchain may impact clinical trials.
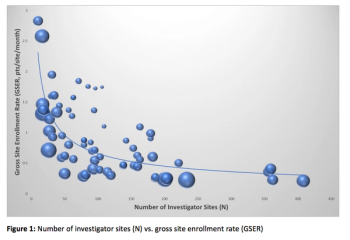
A discussion of the cost and time put into clinical trials.

There are several pharmacokinetic factors and peculiarities that should be considered carefully when designing and running first-in-human (FIH) studies for monoclonal antibodies.



Newest revelations suggest Risk Assessment Categorization Tools are now becoming therapeutic area-specific, as an oncology-based risk library is now surfacing.

BMS executive shares his perspectives on the current biomarker landscape, the role of translational medicine in accelerating this area of study, and how BMS is working to advance biomarker research across their R&D portfolio.

Peter O’Donnell explores potential parallels of the U.S. “right-to-try” debate in Europe.

Examining the unique standards and related challenges when assessing the safety and efficacy of cancer immunotherapy candidates.

Click the title above to open the Applied Clinical Trials May 2018 issue in an interactive PDF format.