
News





Kristy Galante, Director Process and Infrastructure of External Alliances at Janssen, recently spoke about a novel vendor oversight model at ExL’s 8th Clinical Quality Oversight Forum, and will expand on the model in this interview.





Updated employee announcements, business news, awards, and recognition in the industry today.


Case study shows that site activation is a key driver in determining patient enrollment cycle time, which can be minimized by identifying the "sweet spot" of sites to deploy for a clinical trial.

Case study demonstrates that site activation is a key driver in determining patient enrollment cycle time.


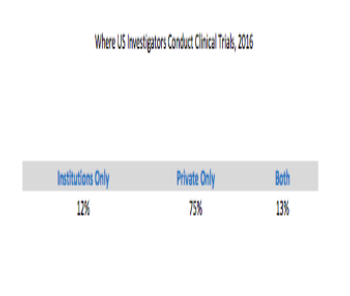
A data analysis focusing on where US investigators conduct clinical trials.


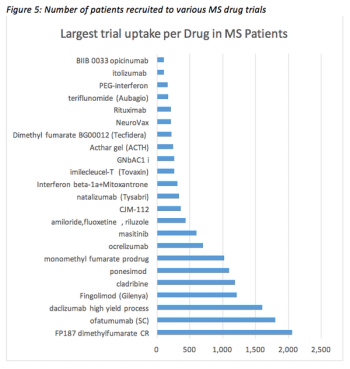
Examining the main challenges in designing and executing MS clinical trials and proposing mitigation strategies that may help alleviate these burdens.

Click the title above to open the Applied Clinical Trials January/February 2018 issue in an interactive PDF format.

2017 has been an exciting year for the clinical trials industry on many fronts, driven by new methods, regulation, and technology, but what is to come in 2018?



Mobile health devices are poised to transform medical care-and with it the medical device market.
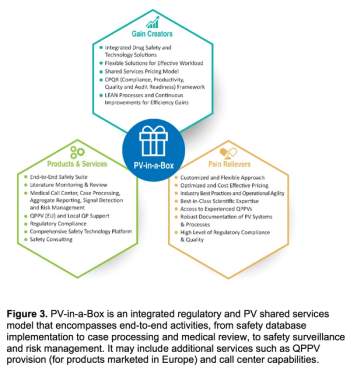
Outsourcing safety responsibilities to functional service providers (FSPs) during clinical trials and post-approval can help small-to-medium-sized enterprises balance their workload and maintain best-practice operations.

Today, a data-driven approach to feasibility assessment can help ensure that a sponsor’s clinical research plan is designed to enroll the right patients, rely on the right investigators, and take place in the right countries for success.


