
Research shows there was no decline in non-COVID related study spending during height of pandemic.

Research shows there was no decline in non-COVID related study spending during height of pandemic.
With Open Payments it is possible for Pfizer to better understand its own investigator usage pattern and how that pattern may differ from the practices of other pharmaceutical companies.
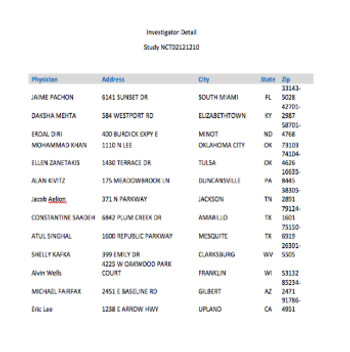
With Open Payments US investigator names and experience will become public knowledge.
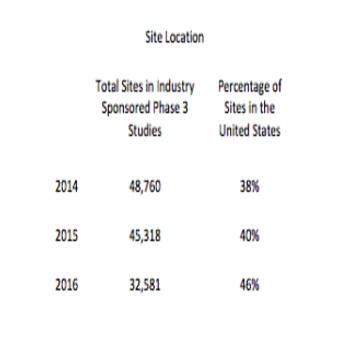
More recent ClinicalTrials.gov data indicate that the United States remains the central location for clinical trial activity.
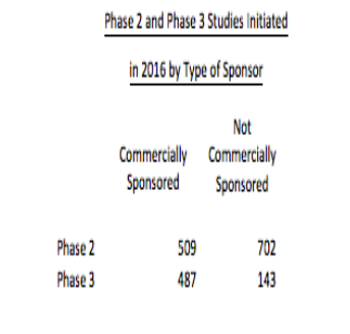
With the pharmaceutical industry looking for new investigators, a non-commercial clinical trial has become an under appreciated source.
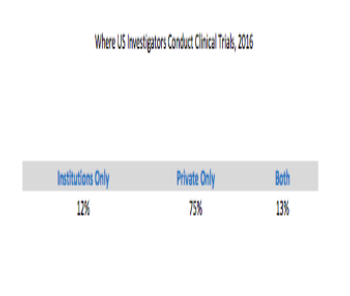
A data analysis focusing on where US investigators conduct clinical trials.
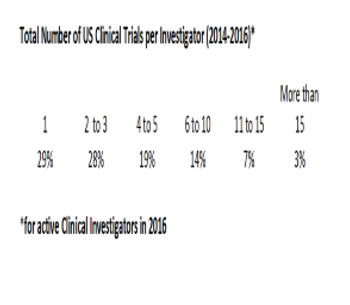
This data analysis takes a look at how much experience US clinical investigators have.
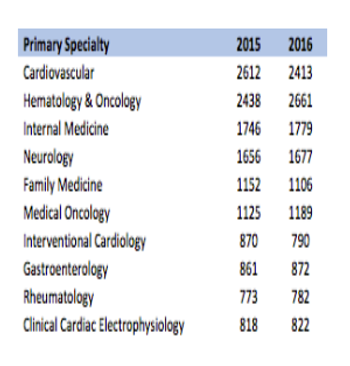
Estimates of active US investigators range from about 20,000 to almost 150,000.
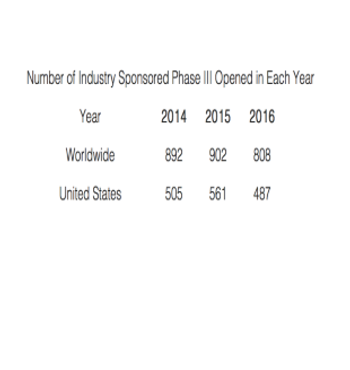
Published data indicate the size and complexity of these Phase III studies has hardly changed over the last few years.
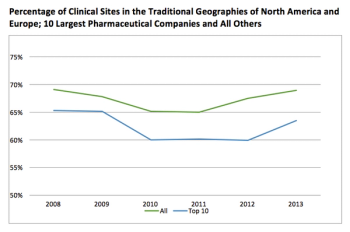
Most clinical trial activity for Phase III trials takes place in the traditional North American and European markets.
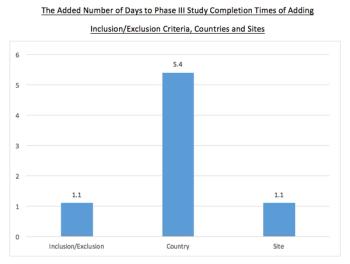
This analysis uses a large dataset to forecast the study completion time costs of specific protocol designs and execution plans.
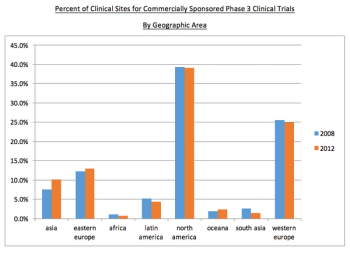
The globalization of clinical trials is the subject of this analysis to find if there is an increase in non-traditional sites.
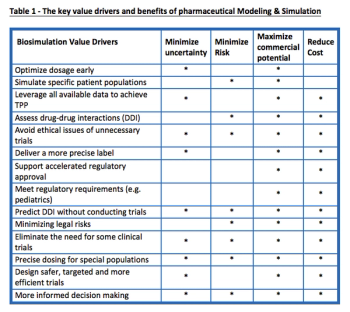
The drug development process has been seen as inefficient and disappointing by those in the R&D space. Model and Simulation provides a potential relief to these issues by delivering significant business, scientific and clinical value to drug developers.
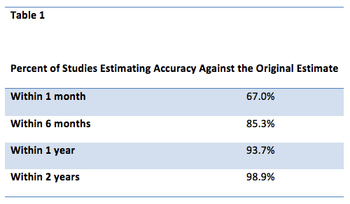
With a unique business model, pharmaceutical companies and their subcontractors need to find every way possible to shorten drug development times consistent with patient safety. The challenges facing commercially oriented organizations developing prescription drugs are substantial.
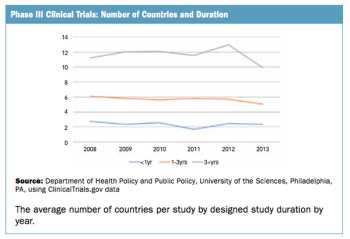
Trend doesn't seem to fall in line with the perception of an increasingly complex trial protocol climate.
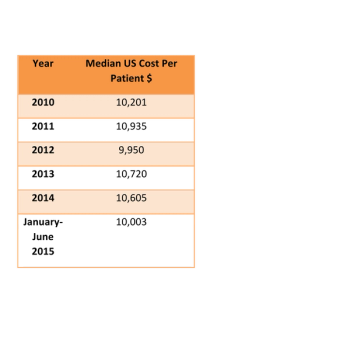
A substantial increase in procedure complexity should be reflected in increasing per patient costs of Phase III clinical trials,which is not true from this data.
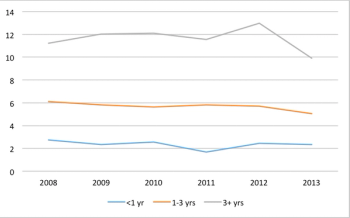
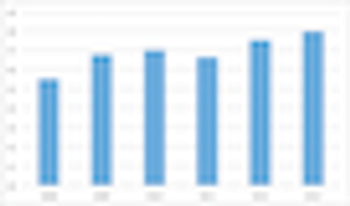
The number of countries used in commercially sponsored Phase III clinical trials has not changed in recent years.
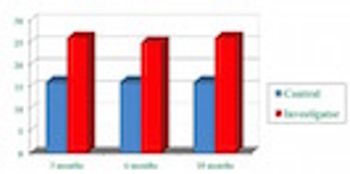
IMS data show the importance of the role clinical investigators play in prescribing.
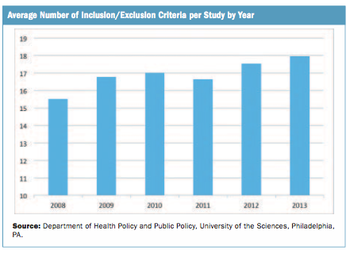
A review of public data indicates a small increase in Phase III inclusion/exclusion criteria per trial over a five-year period.
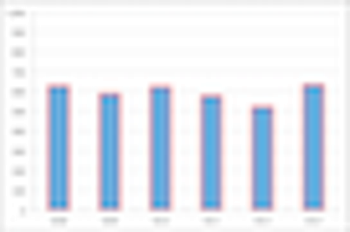
Randomized Clinical Trials (RCTs) have constituted the foundation for new drug approvals for over fifty years.
Few topics occasion the lamentations of clinical trial professionals more than the topic of patient inclusion/exclusion criteria in clinical protocols.
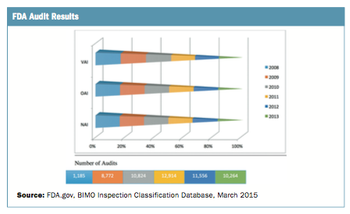
The number of site audits performed has grown significantly.


An increasing amount of public data on clinical trial research has become available, including ClinicalTrials.gov and Open Payments.

ClinicalTrials.gov is a federally mandated database with a large and growing, number of required data fields. Organizations are required to report any study being conducted under FDA auspices. One of the mandated reporting fields is the date that the FDA receives notification of a clinical trial’s initiation, with less than 3% of the studies in the database missing that information.
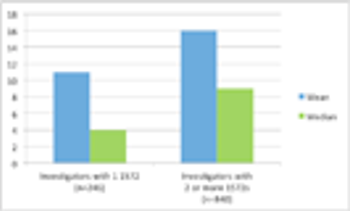
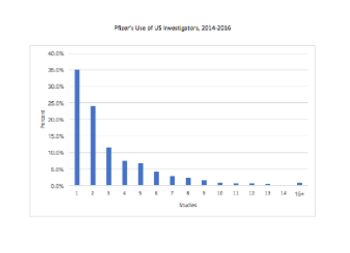
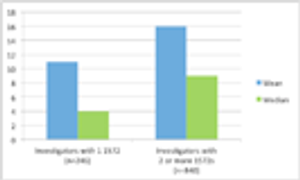
Do 50% of US investigators only conduct one clinical trial? Much of the misunderstanding behind this “one time” claim, which first surfaced in the 1990s, rests on a misunderstanding of the BMIS.

Published: February 28th 2023 | Updated:
Published: April 22nd 2015 | Updated:

Published: May 13th 2015 | Updated:

Published: May 27th 2015 | Updated:

Published: June 26th 2015 | Updated:
Published: August 10th 2015 | Updated: