
Risk-Based Monitoring
Latest News
Latest Videos

More News

Analysis assesses the relative percentage of quality issues detected via SDM that clinical study teams considered critical.

Webinar Date/Time: Tue, Mar 26, 2024 11:00 AM EDT

Webinar Date/Time: Thursday, March 14th, 2024 at 11am EDT | 8am PDT | 3pm GMT | 4pm CET

Webinar Date/Time: Wed, Mar 27, 2024 11:00 AM EDT

Study teams often face challenges in maintaining detailed and accurate documentation of risk signals.

Site and project management teams play major roles in risk-management and monitoring performance.

Webinar Date/Time: Tue, Oct 17, 2023 11:00 AM EDT

Overall rate of significant KRI results for sites located in Ukraine has consistently risen since 2021.

Collaboration and regulation were the watchwords at DIA Global this year, where the ICH E6(R3) guideline, published in final draft form in May, was the talk of the congress.

Study Health Check provides early identification of issues related to protocol deviations and determines objective measures of site-specific versus study-wide performance.

Metric derived from CluePoints central monitoring platform assesses average total cycle time from risk signal creation until closure.

Strategies for intervention—or, ideally, averting potential pitfalls from the onset.

Statistical methods used via this technique in centralized monitoring.

Webinar Date/Time: Option 1: Thursday, May 11th, 2023 at 9am EDT | 6am PDT | 2pm BST | 3pm CEST Option 2: Thursday, May 11th, 2023 at 2pm EDT | 11am PDT | 7pm BST | 8pm CEST

As challenges like regulatory adherence, cost, and timelines become more complex, sponsors are seeking out new ways to incorporate risk-based approaches.

Industry sees high increase in the use of QTLs.

Addressing Operational and Technical Challenges in Home-use Point-of-Care Device Development and Deployment
Webinar Date/Time: Mon, Feb 13, 2023 10:00 AM EST

Study indentifies trends in most commonly used KRIs.

With risk-based quality management (RBQM), the industry is uniquely positioned to amend Murphy’s Law and propose something new—“Everything that can go wrong, we can work to identify and prevent.”

Advancing risk-based auditing one step further by identifying the underlying process deficiencies to enable improved corrective and preventive actions.

Series Part 3—Methods for early detection of risk and summary.


Series Part 1—Introduction and the relationship between QTL and KRI.

COVID-19 may have accelerated the adoption of RBQM, but it is the intrinsic benefits that are cementing its continued use.
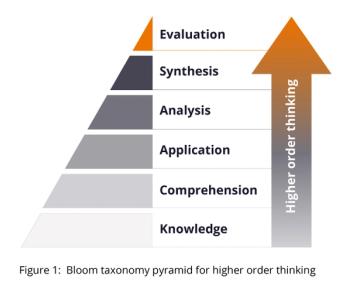
Developing a higher level of critical thinking can create a comprehensive risk story and properly direct mitigations throughout your organization.





