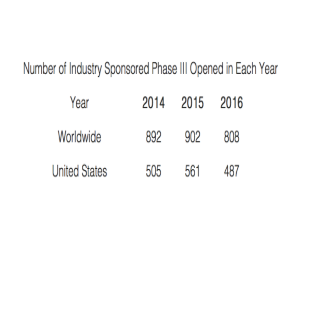
Investigative Sites
Latest News
Latest Videos

More News
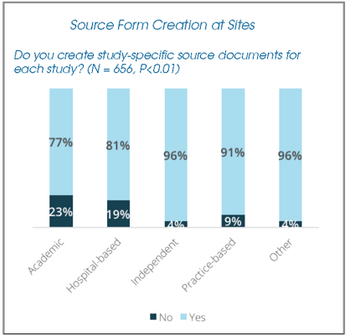
Tanya Bridges and Donna Benson, from two non-affiliated study sites, discuss the burdens of using paper source forms, and their impact on resources and trial execution at study sites.

The nascent and fragmented global community of investigators is showing signs of scaling and maturing.
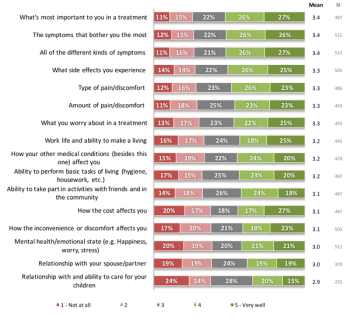
A survey from the Avoca Group examines issues related to quality of clinical trials and trial participation from the patients' view.

Meghan McKenzie, Associate Director and Sr. Clinical Program Lead at Genentech, elaborates on her experiences in the field of patient centricity from the perspective of the sponsor.

The FDA’s Office of Regional Affairs will look to implement their much-anticipated Program Alignment initiative, thus reorganizing the FDA field force in 2017. This new program will alter bioresearch monitoring of clinical research operations.

New study finds that the long-strained relationship between sites and institutional review boards may finally be improving.

A patient-centric approach to a Phase IV trial for an MS treatment resulted in lessons that can be applied to future therapeutic studies for this and other rare diseases.

Work burden and performance hurt by technology incompatibility.

Over the last 30 years, the process of clinical research and clinical trials have undergone a revolution. However, despite these changes investigator meetings have not evolved.

Survey drills down on the preferences and experiences of clinical trial site personnel in critical function areas such as site initiation and monitoring, training, sponsor interaction, and patient recruitment and retention.

On Wednesday, DrugDev is debuting its Site Activation Module at the SCOPE Summit. The company believes the module will greatly improve all tasks of site activation, from protocol feasibility, site identification and selection, to contracts and the collection of site regulatory documents.

While several studies and surveys have examined the relationship between sponsors and CROs, there has been little analysis of CRO-investigative site relationships. This study uncovers useful learnings on the positive and negative experiences of investigators in their direct dealings with CROs.
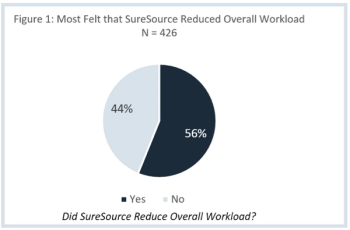
Clinical Ink, an eSource solutions provider, offered Applied Clinical Trials raw data from its Site Impact Survey for analysis. This article will delve into this data to uncover breakthrough trends regarding the utilization of eSource on workload reduction at study sites.
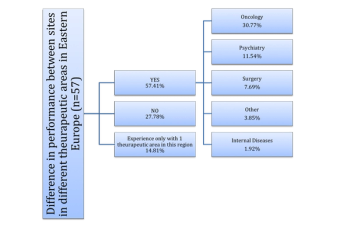
Survey of pharma and CRO clinical trial professionals attempts to answer whether Eastern European countries are still considered attractive destinations for clinical research.

This article will describe my experiences in acquiring new clinical trials from the study site’s standpoint.

Study takes rare look at the financial and resource burden for sites in managing regulatory compliance.
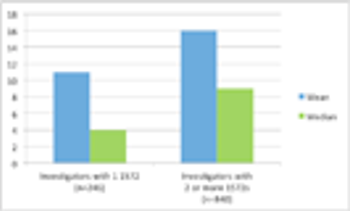
Do 50% of US investigators only conduct one clinical trial? Much of the misunderstanding behind this “one time” claim, which first surfaced in the 1990s, rests on a misunderstanding of the BMIS.
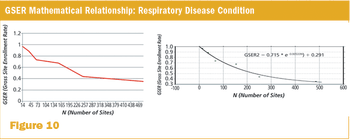
Building a structure for managing study operations through linking trial- and site-level forecasting.
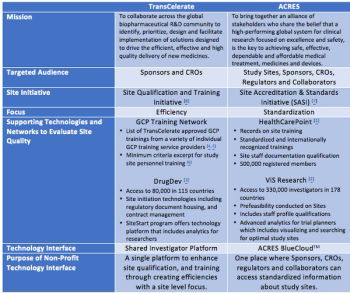
Over the past several years, there has been a rise in nonprofit organizations that focus on addressing the challenges in clinical research.
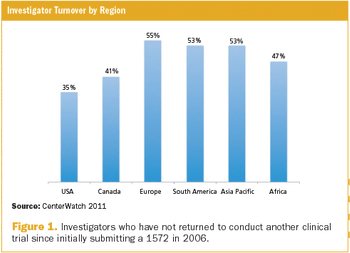
The sharp rise in ongoing clinical research studies is driving demand for greater participation in research by physicians as well as by patients.1
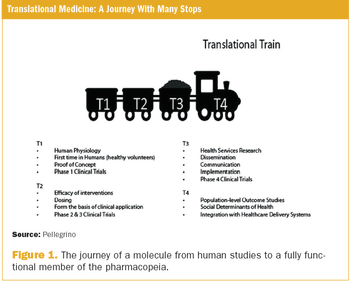
The organization of healthcare is changing rapidly. The healthcare delivery system is increasingly powered by payers and regulators, and this directs both clinical medicine and drug development. Partly because of this change, the drug development process has been heavily scrutinized, and a great emphasis has been placed on more efficient translation of basic science into useful medicines.
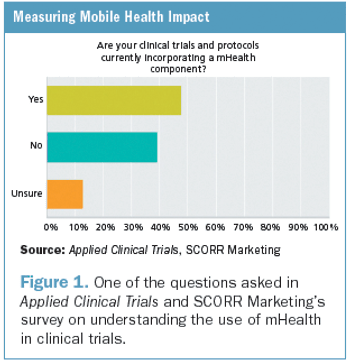
After a five-year review of clinical trial quality measurement research and practices, we have concluded that clinical trial quality measurement does not meet current scientific standards

Patient enrollment for clinical trials is not a numbers game.
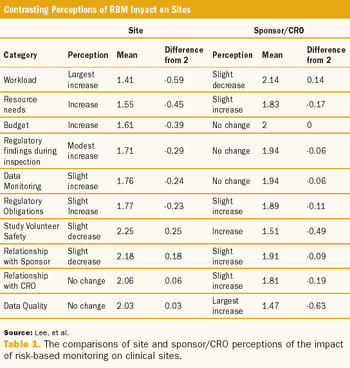
Sponsor-site alignment on risk-based approaches is critical if clinical trial standards are to reach new levels.

The integration of pre-screening all patients for eligibility in oncology studies.