
Supporting real change in clinical trials is more than just lip service-it’s putting the information out there transparently for all stakeholders to measure and make decisions.

Supporting real change in clinical trials is more than just lip service-it’s putting the information out there transparently for all stakeholders to measure and make decisions.

Jill Wechsler details new FDA policies to streamline drug development, including the design of “seamless” trials.

Rho offers their expertise on why it is important to have a pre-IND meeting to ensure a successful IND.

With the EU’s new General Data Protection Regulation coming into force in May this year, the impetus for life sciences firms to cement their data management strategies has increased.

An overview of all the drug approvals that were secured in 2017.

Under pressure to meet tight deadlines for reviewing and approving a growing volume of applications for new drugs, generics, and medical products, FDA is rejecting submissions that are incomplete or unsatisfactory right from the start.

The FDA published a final rule on FDA’s standards for accepting data from clinical investigations for medical devices on February 21.

Jill Wechsler details the two chief reasons why clinical trial quality and efficiency has improved in recent years.

The FDA's new draft IVD Guidelines will need to be factored into how pharma clinical trial sponsors use IVDs in a clinical trial, study design, timeline for protocol development, IND submission, and study initiation.

Now the challenge to FDA and to sponsors is to maintain the high level of support for research, discovery, and regulatory flexibility underpinning these gains, writes Jill Wechsler.

In this interview, Austin Speier, VP of Emerging Technologies at Precision for Medicine, will elaborate on these draft guidance documents.

The agency is moving to smooth the pathways for orphan drugs, genetic therapies, and other scientific advances to yield more transformative medicines.
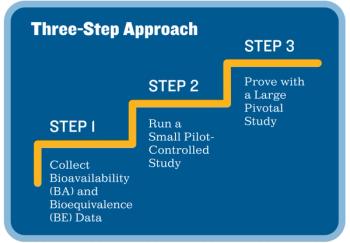
Sponsors can streamline the design and execution of their clinical trials by following this three-step approach.

Jill Wechsler talks about biosimilars and the requirement of clinical trials in her recent blog.
This article will dive into the details of FDA’s movement in mHealth, analyze FDA’s approach, and assess how this movement impacts the use of mHealth in clinical trial settings.

New legislation aims to expand regulatory acceptance of patient data from healthcare systems and observational studies.
This brief overview of Kazakhstan presented by Vlad Bogin will help you get acquainted with this emerging clinical trial location.

There is a critical need to rethink standards of evidence and of the reliability of information used to make regulatory decisions. According to the FDA, this involves placing greater reliance on data from sources outside traditional clinical studies and because of these new tools for collecting the data, the FDA needs to adapt as well.

The EU is launching a major transition within their legislation for medical and in vitro diagnostic devices. This transition is important and should include assessing the financial viability of the pipeline, expanding data collection, and locking in soon-to-be-scarce notified body resources.

The agency is encouraging drug companies to adopt innovative research methods and development tools-and showing more flexibility in approving therapies that have taken non-conventional paths.

With the rise in deaths, injuries, and treatment costs related to the abuse and misuse of opioid painkillers, the biomedical research community is seeking more effective and informative methods for testing and evaluating new pain medicines. Jill Wechsler reports.

The FDA ruling that exempts biosimilar makers from waiting an extra six months after approval to distribute a new product should help overcome delays in future biosimilar sales, writes Jill Wechsler.

With the new FDA commissioner officially in, the FDA begins the considerable task of implementing key "Cures" initiatives.

The majority of pregnant women are prescribed treatments that can include antibiotics, antivirals and more. However, little to no information is available about the appropriate dose to prescribe or the potential adverse fetal effects.

Policy makers, sponsors and regulators are taking steps to promote alternative study formats and methods that go beyond randomized clinical trials.