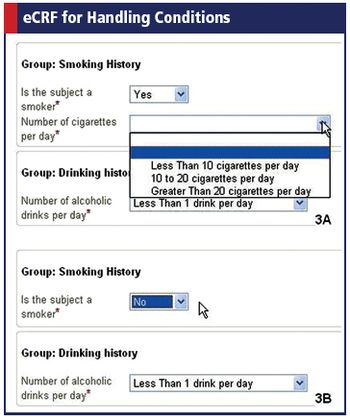
The group's latest ODM release strengthens global reach and lets researchers define conditions in eCRFs.

The group's latest ODM release strengthens global reach and lets researchers define conditions in eCRFs.

A review of Euromed Communications' recently published: Paediatric Clinical Research Manual

A discussion of the recent initiatives for applying advanced technologies in real world settings that have the capability to improve drug research, increase subject safety, and reduce development costs, which takes into account the collaborative efforts among government, academia, industry, and patient groups that are necessary to achieve these goals and translate new technological discoveries into clinical practice.

Favorable cost, research quality, and timeline measures are attractive to overseas sponsors.

Rise in foreign studies requires attention to ethical issues, investigator training.

EMEA gives EU six months to respond to highly technical draft guidance on viral safety of biotech meds.

Less stringent requirements in the European Union result in faster medical device approval times.

A patient's agreement to take part in a clinical trial is a legal contract, which consumer law requires to be expressed in plain language.
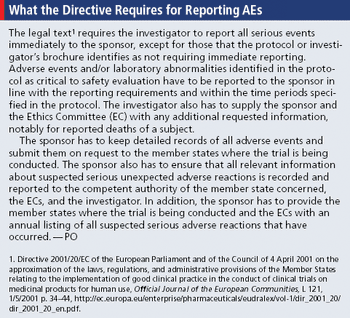
EU helps ease ambiguity of reporting process with recent published guidance.

Software vendors can help sponsors ensure clinical trial data are accurate, reliable, and authentic.

Why research in Europe has declined since the implementation of the Clinical Trials Directive.
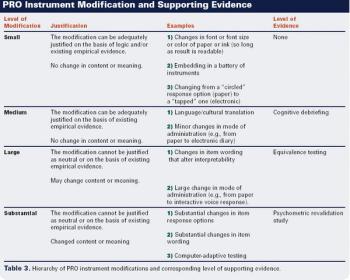
What the agency requires to support the selection of patient reported outcome instruments.

Agency seeks to calm critics by improving subject protection, while also streamlining research oversight.

Payers seek more comparative drug data from sponsors and independent researchers.

European Medicines Agency adopts new approach to drug development process.

This new course aims to provide clinical research physicians with good clinical practice knowledge.
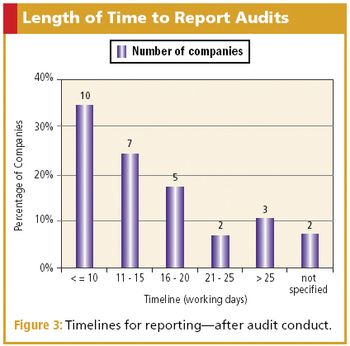
CQA benchmark survey finds companies use a similar method to conduct and report audits.
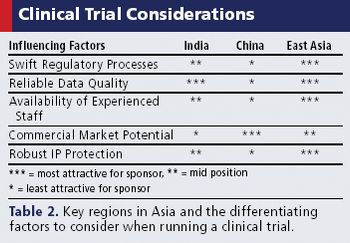
Rapid recruitment, potential cost savings, and investigative sites are just a few of the factors attracting sponsors to the region.
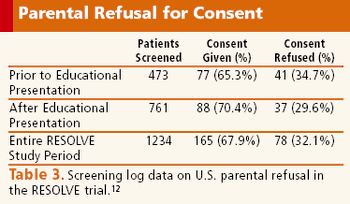
A successful investigative team shares their ideas on improving enrollment in children's research.

New Q&A guidance attempts to clear up any confusion about new Directive rules.

The 2005 guidance reinforces adoption of medical imaging for cancer endpoints.

New EU legislation heralds a difficult funding environment for nonprofit research centers.

A growing number of sponsors and regulators are recognizing that accredited organizations are more likely to be compliant.

EU Council supports pediatric medicines proposal, but adds some changes.

The much anticipated list features initiatives in biomarkers and research productivity.