
Tom Cowen, head, healthcare, life sciences, Conga, explains how biotech startups use scalable contract management platforms to efficiently manage clinical trials, support CRO partnerships, and accelerate drug development pipelines.

Tom Cowen, head, healthcare, life sciences, Conga, explains how biotech startups use scalable contract management platforms to efficiently manage clinical trials, support CRO partnerships, and accelerate drug development pipelines.

Tom Cowen, head, healthcare, life sciences, Conga, shares how leading pharmaceutical companies are using Contract Lifecycle Management to streamline global studies, reduce onboarding times, and standardize clinical trial processes.

Tom Cowen, head, healthcare, life sciences, Conga, outlines how AI-driven contract management helps pharmaceutical companies simplify global clinical studies, reduce risks, and accelerate trial timelines.

Tom Cowen, head, healthcare, life sciences, Conga, explains why investigator onboarding creates significant delays in clinical trials and how smarter contract management can help sponsors accelerate study start-up.

Paul Shawah, EVP, strategy, Veeva Systems and Richard Staub, president, R&D solutions, IQVIA, discuss how their partnership is evolving to support decentralized and hybrid clinical trials by combining advanced technology with operational expertise to improve patient engagement, streamline processes, and accelerate study timelines.

Paul Shawah, EVP, strategy, Veeva Systems and Richard Staub, president, R&D solutions, IQVIA, explain how their partnership is improving efficiency and data management in clinical trials by integrating best-in-class technology with expert services to benefit sponsors and accelerate drug development.

Paul Shawah, EVP, strategy, Veeva Systems and Richard Staub, president, R&D solutions, IQVIA, explain how their partnership is accelerating global clinical trials by streamlining processes, improving data management, and enabling sponsors to bring new treatments to patients faster.

Paul Shawah, EVP, strategy, Veeva Systems and Richard Staub, president, R&D solutions, IQVIA, discuss how their partnership leverages cutting-edge technologies and integrated services to improve data accuracy, streamline workflows, and drive greater efficiency in global clinical trials.

Full patient enrollment in REVEAL-1 (NCT06812325) and REVEAL-2 (NCT06625398) trials mark a key milestone for VRDN-003, Viridian’s promising new therapy for thyroid eye disease.

Paul Shawah, EVP, strategy, Veeva Systems and Richard Staub, president, R&D solutions, IQVIA, discuss how their global partnership is streamlining workflows, integrating data and AI, and driving better outcomes for patients.
Preliminary Phase IV trial results show that Moderna’s 2025–2026 Spikevax formula boosted neutralizing antibodies more than eight-fold in high-risk adults and older populations against COVID-19.

The Phase II trial (NCT07145229) will evaluate the safety, efficacy, and pharmacodynamics of ABP-745 compared with placebo and standard colchicine therapy in over 200 patients with acute gout flares.
Interim results from the ongoing Phase IV LOTUS trial showed that Daybue delivered sustained behavioral improvements and manageable gastrointestinal outcomes in Rett syndrome patients over one year.
Results from the Phase III STRIDE-13 trial (NCT06177912) showed that Merck’s Capvaxive was noninferior to PPSV23 in children and adolescents aged 2 to <18 years who are at increased risk of pneumococcal disease due to chronic medical conditions.
The FDA-approved therapy is now included in National Comprehensive Cancer Network clinical guidelines as a treatment option for both pediatric and adult patients with aggressive H3 K27M-mutant diffuse gliomas, offering a new standard of care for recurrent disease.

Results from the two-year AVONELLE-X extension study (NCT04777201) and the Phase IIIb/IV SALWEEN trial showed that Roche’s Vabysmo demonstrated durable vision improvements, extended dosing potential, and reliable safety treating wet age-related macular degeneration and polypoidal choroidal vasculopathy.

The designation supports expedited development of SAR402663, Sanofi’s one-time gene therapy designed to reduce treatment burden and slow vision loss in patients with wet age-related macular degeneration.
Results from a Phase II trial (NCT06449209) showed that BioNTech’s and Bristol Myers Squibb’s pumitamig in combination with standard chemotherapy demonstrated encouraging anti-tumor activity in patients with extensive-stage small cell lung cancer.
Results from the Phase III ASCEND trial (NCT05882877) showed rocatinlimab maintained long-term efficacy with a favorable safety profile, offering potential for extended dosing intervals in adults with moderate to severe atopic dermatitis.
Results from the Phase III FLAUR2 trial (NCT04035486) showed that Tagrisso combined with pemetrexed and platinum-based chemotherapy achieved a median overall survival of 47.5 months in patients with locally advanced or metastatic EGFR-mutated non-small cell lung cancer.
Results from the Phase III HARMONi trial (NCT06396065) showed that ivonescimab demonstrated a median overall survival rate of 16.8 months in patients with EGFR-mutated, locally advanced or metastatic non-squamous non-small cell lung cancer.
Results from a Phase III trial (NCT07069309) demonstrated that the LP.8.1-adapted Comirnaty vaccine generated a robust fourfold increase in neutralizing antibodies of COVID-19.
Phase IIa trial (NCT06413537) results show SPG601 improves neurophysiological and cognitive measures in patients with Fragile X syndrome.

Results from the Phase II EPCORE NHL-6 trial (NCT05451810) showed that Epkinly demonstrated an overall response rate of 64.3% in patients with relapsed or refractory diffuse large B-cell lymphoma.
Designation supports the potential of olomorasib plus Keytruda to address unmet needs in the first-line treatment of patients with KRAS G12C-mutant non-small lung cancer with high PD-L1 expression.

Results from the Phase IV V-DIFFERENCE trial (NCT05192941) found that 85% of patients on Leqvio reached guideline-recommended LDL-C targets versus 31% on placebo at 90 days.
Results from the Phase III CORALreef Lipids trial (CT05952856) showed that patients treated with enlicitide decanoate for hypercholesterolemia demonstrated statistically significant and clinically meaningful reductions in LDL cholesterol.
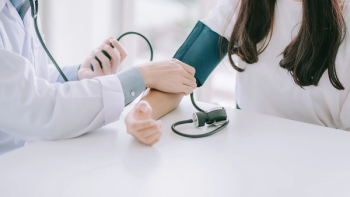
Results from the Phase III BaxHTN trial (NCT06034743) showed baxdrostat achieved statistically significant and clinically meaningful reductions in systolic blood pressure in patients with hard-to-control hypertension.

The ZENITH outcomes trial will enroll 11,000 patients worldwide to evaluate twice-yearly zilebesiran, aiming to improve long-term blood pressure control and reduce cardiovascular risk in uncontrolled hypertension.

The Phase III VICTOR trial (NCT05093933) evaluated Verquvo in patients with stable, well-treated chronic heart failure and reduced ejection fraction without a recent worsening heart failure event.

Published: November 30th 2023 | Updated:
Published: January 22nd 2024 | Updated:

Published: February 17th 2025 | Updated: February 17th 2025
Published: January 22nd 2024 | Updated:

Published: September 17th 2024 | Updated:
Published: February 19th 2024 | Updated: