The perfect storm of industry growth, opportunity, and demand is translating to solid salary and satisfaction levels among clinical trial professionals, according to new survey.

The perfect storm of industry growth, opportunity, and demand is translating to solid salary and satisfaction levels among clinical trial professionals, according to new survey.

Using venture philanthropy to get promising drug candidates past “the valley of death”-and supported by advanced scientific technology-rare disease patient organizations have moved beyond being just hopeful influencers, to now becoming powerful forces for change.

Greenphire's Chief Commercial Officer predicts 2020 will continue the momentum towards greater technology adoption and patient focus.








New recommendations offer guidance on overcoming legal, regulatory, and practical hurdles.

Involving patients more meaningfully in the industry's activities means getting more serious about their emotional wellbeing.

Trial participants are willing to share data to improve their lives and the lives of others, but they also request that data and clinical trial results are shared back to them.
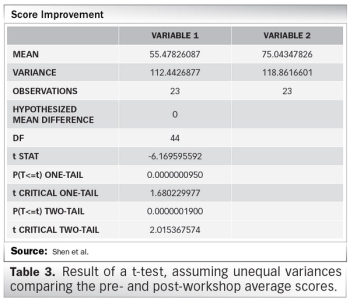
Survey results from Clinical Research Malaysia’s GCP refresher workshop.

Survey data finds that physicians aren’t very familiar with the intricacies of nucleic acid-based therapies.

Gains in FDA approval of new drugs and biologics last year are based on limited clinical trials and accelerated review programs.

How new technology can impact cardiac imaging in oncology clinical trials and have broader implications for patient safety.

Social media uses transparency to highlight alleged gaps in clinical trial transparency and offers a more powerful approach to health campaigners.

Click the title above to open the Applied Clinical Trials January/February 2020 issue in an interactive PDF format.

David Arthur, CEO of Salarius, discusses efficacy, collaborating with nonprofit institutes to obtain funding, and garnering patient support to increase recruitment in clinical trials.
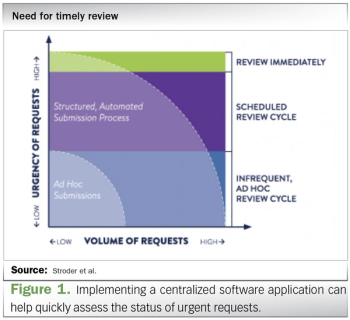
ViiV Healthcare outlines techniques and tips for implementing a technology-based process for handling compassionate use requests.

A compilation of recently released news briefs that pertain to the clinical trials industry.

With the biopharma industry making strides in gene therapies by focusing on patients with specific genetic traits, Karmen Trzupek, Director of Clinical Trial Services at InformedDNA, discusses how to address the presented challenges of this approach.

The EMA is going to take a rather bolder approach to the use of data than in Germany, judging from major statements just out at the end of January.




