
News

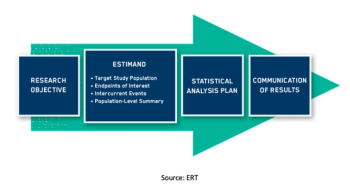
With the final approval and release of ICH E9 (R1), clinical trial sponsors should prepare to include estimands in their protocols as well as their statistical design documents and study reports.










With the coronavirus now spread across northern Italy, the EU is assembling its forces to slow the virus with an aid package worth $250 million.





Sponsors conducting trials in China are facing delays, while efforts to test potential antivirals and vaccines against COVID-19 are having enrollment difficulties.

Teenagers with cancer could benefit from a recommendation to lower the age barrier so they can take part in clinical trials says a proposal from the Fostering Age Inclusive Research Trials Initiative.
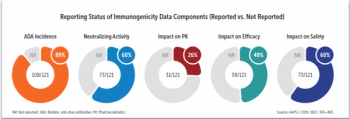
How modeling and simulation technology can predict and better manage immunogenicity, a key challenge for biologics drug development.

The latest happenings in the industry, all in one place.





While “Big Data” is a great buzzword in management circles, small data (with big problems) never gets much attention.



Analysis of over 330,000 Phesi trial protocols shows predicting future enrollment performance by extrapolating data is flawed.
