
News

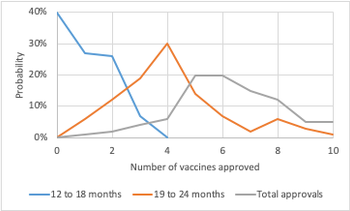
Policymakers should break with drug and biotech norms and apply risk-based portfolio simulations to understand the global portfolio of COVID-19 vaccines.
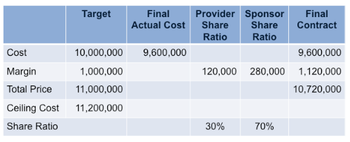
In a complex world of global clinical trials where execution is riddled with uncertainty, how should sponsors and providers approach and achieve a balance of risk and reward?




A discussion of how the coronavirus pandemic will have a multitude of impacts on the conduct of trials, as well as on the industry itself.

A global survey of clinical trials sites, conducted by insights company Clinical SCORE, revealed how deep the effect of the COVID-19 pandemic is and how sponsors can help sites stay the course.



The latest business and people happenings, and COVID-19 response, from around the industry.




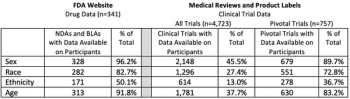
Demographic Disparities in Patient Samples for Drugs and Biologics Approved by FDA Between 2007-2017
Diversity in clinical trials is an important part of developing new medications that are safe and effective for all potential patients, understanding the demographic disparities within them will provide several benefits to the field.

Industry experts weigh in on how much traditional approaches in clinical operations need to change to meet new expectations for clinical delivery.
Lockstep with regulatory guidance on conducting trials amid crisis, here are four key actions sponsors can take to minimize disruptions.






Findings from a survey of 363 clinical trial sites showing the profound effect of the coronavirus pandemic.




The latest FDA and EMA guidelines permit sites, sponsors, and CROs to adjust their operations to meet changing conditions for ongoing trials, including some concerning the safety of participants, that must be met in order for new solutions to be considered.

