
News



Clinical trial stakeholders weigh in on the major shifts unfolding in the era of COVID-19, as efforts move away from the traditional site-based model.

Findings from an OCT Clinical survey of researchers from 61 sites in Russia to gain the perspective of an investigator regarding clinical trials during the COVID-19 pandemic.






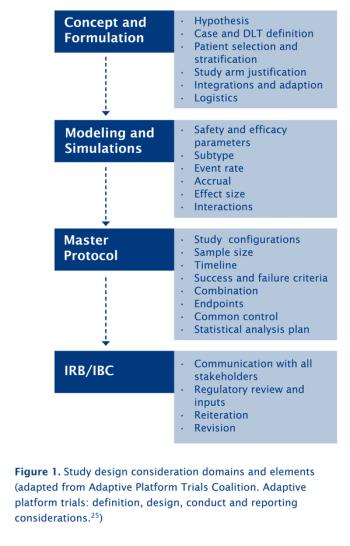
IMPs for immunotherapeutics require a different mindset, effective strategies, and meticulous planning, among other things, says KCR Trial Execution and Consulting.




Now that the industry has gotten some experience with CDISC standards, teams are aiming to get more from their standardization efforts.



Outlining the five critical hurdles faced by clinical teams conducting studies in these nations amid quarantine and other restrictions.
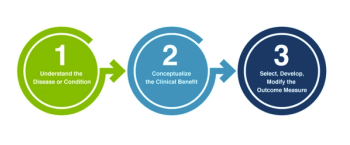
The four new methodological Patient-Focused Drug Development guidance documents the FDA is currently developing for the industry that incorporate patient experience data into drug development, summarized.

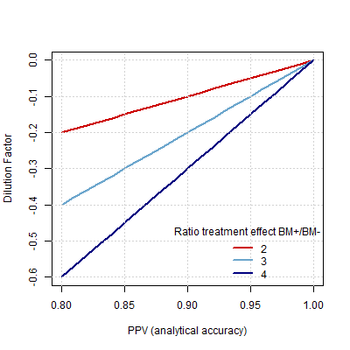
The development and qualification of biomarkers are keys to the future of drug development and precision medicine, particularly in oncology.


Challenges faced by researchers conducting clinical trials in low- and middle-income countries are examined.
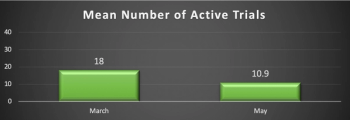
Results from a Clinical SCORE study shows investigators and other site staff are begging sponsors and CROs to match their tenacity to soldier on.


COVID-19 response is leading to new measures being introduced in many countries, including Russia, Europe and CIS countries, especially when it comes to the interaction of research centers with CRAs.
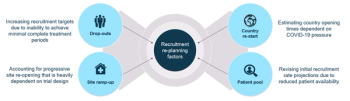
Sponsors need to develop a strategy on how to evaluate the impact of the pandemic on the re-start of halted trials.

Tufts Center for the Study of Drug Development and Biogen recently conducted a study to inform growing interest in improving diversity of clinical trial participation. The results of this research provide insights into increasing the community of minority investigators and study staff and presenting greater access to clinical trials among minority study volunteers.

