
News








John Rigg, senior principal, Predictive Analytics, Real World Solutions at IQVIA, discusses how patient disease modeling and diagnostic prediction is made possible with artificial intelligence and machine learning.


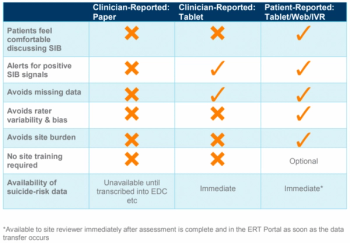
During clinical trials of pharmaceutical treatments, sponsors and CROs need to detect possible suicidal ideation and behavior related to their drug during baseline assessments, and throughout treatment as well as during the follow up phases.

Non-reporting has attained a high profile in Europe, partly because of the EMA's attempts to tackle the problem, and partly because of a highly-visible “name-and-shame” social media campaign.


While the clinical trials industry has always been a slacker in innovation, we'll soon start seeing automation and artificial intelligence significantly impacting the way biopharmaceutical enterprises run clinical trials, says Moe Alsumidaie.







Therapies aimed at correcting genes that cause diseases are gaining ground in biopharma, some are even specializing in specific mechanisms of action by targeting and modifying RNA through temporary and ongoing treatment. CEO at ProQR Therapeutics, Daniel de Boer, discusses how they are advancing RNA therapies in retinal disorders.



The latest industry happenings, all in one place.




