
Gelenberg brings years of expertise to EDC/eClinical company.

PAREXEL enhances early phase clinical development offering for central nervous system studies.

Allon uses Nextrials’ Prism to go from last patient visit to results in under seven weeks.

Covance announced today that it has relocated and significantly upgraded its clinical pharmacology research unit in Evansville, Indiana.

Innovative system gives clients real-time access to data, offers secure data warehousing and expedites data set conversions for electronic regulatory submissions

Oncology-focused CRO and research network announces its Oncology Data Warehouse includes information from over 110,000 oncology patients.

Clinical Researchers Present Promising Results for VX-770, an Oral Investigational Agent that Targets a Defective Protein Responsible for Cystic Fibrosis.

OpenClinica, an open source EDC system, to hit the Italian market thanks to a new partnership.

Dr. Robert Leary will discuss computational methods for model evaluation; Dr. Mike Dunlavey will present simplified programming approaches of population model user interfaces.

Good Products launches the Clinical Technology Consulting division to facilitate biopharmaceutical sponsors' and CROs' use of clinical technology.

Growth in adoption of CDISC standards evident at CDISC European Interchange in Copenhagen.

Research shows that tech-savvy pensioners make up the most compliant demographic.

DSMB Charter Template

ICREL allows you the opportunity to give your input through a survey about the EU Clinical Trials Directive.

University of Manchester researchers are working on a more effective treatment for TB.

ClinPhone shows commitment to Good Clinical Practice by completing the latest Medicines and Healthcare Products Regulatory Agency inspection.

Company integrates leading medical imaging technology with image management and reporting system provided by Digisonics.
Insurty gets ready to meet FDA’s requirement that regulatory submissions be in eCTD format.

A new initiative aims to identify new methods to make the clinical trials process more efficient and effective.

A new project focuses on developing Africa's legal and regulatory framework for health research.

DIA expands into India with a new office and advisory council.

Industry news tidbits from around the world.

Industry news focusing on the people and organizations who work in the clinical trials profession.
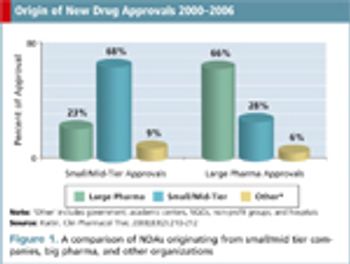
CROs must adjust their practices to cater to small/mid-tier pharma instead of simply servicing big pharma.

December 2007 BPA

2008 Buyers Guide

Therapeutically focused contract research organization to implement Medidata Rave for sponsors to streamline global clinical trials.

New sourcebook offers valuable insight into the clinical trials industry.

Comprehensive data warehouse boosts ACORN's information portfolio.

Synarc adds two new members to its team, strengthening the company’s oncology capabilities.