
The Clinical Data Interchange Standards Consortium (CDISC), announced that the Food and Drug Administration (FDA) selected CDISC, via a formal RFP process, to provide training to FDA reviewers of regulatory submissions.

The Clinical Data Interchange Standards Consortium (CDISC), announced that the Food and Drug Administration (FDA) selected CDISC, via a formal RFP process, to provide training to FDA reviewers of regulatory submissions.

Nextrials, Inc., a clinical research software and services company, announced the addition of chief technology officer, Robert Barr, and vice president of global sales and marketing, Michelle Dockhorn, to its executive staff.

ACRP devotes its September issue to articles related to the importance of trust and building confidence among the public in the clinical research enterprise.

Tripos, a provider of drug discovery informatics products and services, and Pharsight Corporation (NASDAQ: PHST), a provider of software, strategic, and regulatory services designed to optimize clinical drug development, announced they have entered into a definitive agreement for Pharsight to be acquired by Tripos for approximately $57 million in cash.

Kforce Clinical Research, a division of Kforce, Inc. (NASDAQ: KFRC), has won Merck & Co. Inc.'s "Outstanding Operational Award in Clinical Development" as part of Merck's 2007 Supplier Recognition Awards.

Exco InTouch (www.excointouch.com) a mobile messaging provider for the pharmaceutical industry, announces that it has partnered with US-headquartered MMG (www.mmgct.com/mmg) an international industry leader in patient enrollment for clinical trials to enhance the efficacy of patient recruitment and retention services for pharmaceutical sponsors.

iCardiac Technologies, Inc., which develops and implements advanced cardiac safety biomarkers, today announced the successful completion of the industry’s largest validation study for an automated ECG analysis technology.

QPS' clinical research unit, Bio-Kinetic Clinical Applications, announces a joint collaboration with Quintiles that creates an integrated Cardiac Safety Service for sponsors by incorporating clinical conduct, core ECG laboratory support, independent cardiologist and medical monitor oversight, pharmacokinetic and statistical analysis, and reporting of cardiac safety trials.

Phase Forward (NASDAQ: PFWD), a provider of data management solutions for clinical trials and drug safety, today announced that it has acquired privately held Clarix LLC, a provider of fully Web-integrated interactive response technology (IRT), for $40 million in cash.

Rho, Inc., and the National Institutes of Health (NIH), is pleased to announce that the National Institute of Allergy and Infectious Diseases (NIAID) has awarded Rho a six-year, $38.9 million contract to provide biostatistical, data management, and safety services to the Immune Tolerance Network (ITN).

Metrics Inc. now offers microbiology-testing services to the contract pharmaceutical industry
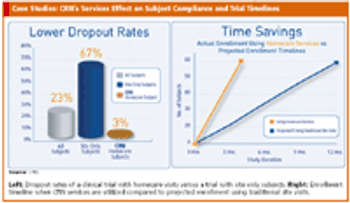
Specialty at-home and alternate-site testing services offer solution for subject recruitment issues.

Health Market Science (King of Prussia, PA) is offering a new data-driven approach to site selection for clinical trials. With nine years experience under its belt with its physician masterfile and claims data
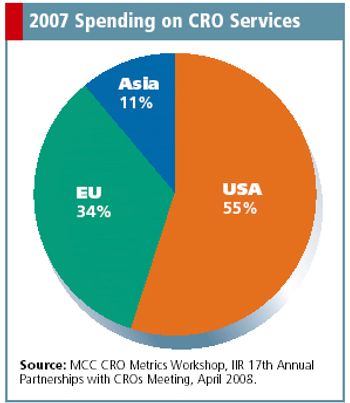
Connecting U.S. sponsors and foreign CROs in an increasingly outsourced market.

Industry news focusing on the people and organizations who work in the clinical trials profession.

New technology has led to a transition from pens to digital signatures and it is becoming a part of everyday life for the pharmaceutical industry.

Inc. magazine ranked Coast Independent Review Board No. 2356 on its 27th annual Inc. 5000 list of the fastest-growing private companies in the U.S.

Biomedical Systems, a global provider of centralized diagnostic testing for the pharmaceutical and medical device industry, recently announced the promotion of Sam Dranoff to Vice Present of Business Development, Imaging Services.

IDIS, a leader in the development and implementation of pre-launch Named Patient Programs (NPPs), announces the opening of a new regional office in Iselin, New Jersey, USA.

ProTrials Research, Inc.TM, a leader in the clinical research organization industry, today announced the company made the 2008 Inc. 5000 list of the fastest-growing private companies in the United States.

The Foundation for Improving Data Quality (FIDQ) recently held its first meeting in Washington, DC, to outline future efforts to refine methodologies that increase the precision of subjective endpoints used in clinical trials.

New data from PAREXEL International Corporation, a leading global biopharmaceutical services organization, reveal that U.S. marketing applications for new molecular entities (NMEs) surged 33 percent in 2007.

AmeriStart announced that it has signed a sales, marketing, and business development services contract with Factory CRO, a leading international clinical research organization that specializes in conducting medical device studies for FDA and EU approvals.

MedNet is proud to announce its inclusion on the INC 5000 list of fastest growing private companies for the second straight year.

This is Applied Clinical Trials' enewsletter, CRO News, which provides a forum for the industry to discuss the latest alliances, financial information, and business deals and developments, as well as people news.

Covance and Eli Lilly announced a strategic collaboration earlier this month representing an innovative solution between a CRO and pharmaceutical company in response to current industry R&D productivity challenges.

PAREXEL International Corporation (NASDAQ: PRXL), a global biopharmaceutical services organization, today announced the successful closing of the acquisition of ClinPhone plc, a clinical technology organization.

Good Products, a leading provider of Enterprise Content Management (ECM) solutions for the pharmaceutical and biopharmaceutical industries, announces the availability of its new Validation Project Management tool ValMan.

Omnicare Clinical Research announced today that it has expanded its global footprint to include an office in Sweden.

Velos, Inc., the resource for clinical trial management information systems for large investigator sites, today announced it will cross the 50 percent market penetration threshold by year end.