
Online survey allows clinical research professionals to share their experiences with the European Clinical Trials Directive.

Online survey allows clinical research professionals to share their experiences with the European Clinical Trials Directive.

The Center for Information and Study on Clinical Research Participation (CISCRP) recognized PharmaNet as this year?s award recipient for ?Supporting the Development of Educational Materials.?

Lawke Links, Cordium Links new joint venture with a Chinese organization, will bring a full range of ECG core lab services to China.
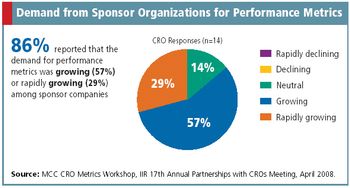
Metrics Champion Consortium standardizes CRO performance metrics across the board.

Symbio LLC, a full service contract research organization, has announced the opening of a new Phase I Clinical Unit in Michigan City, Indiana.

A Russian and Pakistani CRO Announce Network Alliance for Expansion of Global Clinical Trials Services

ICON Medical Imaging Strengthens Cardiovascular Expertise with New Cardiac Scientific Advisory Board Brings together global experts from University of Oxford, Harvard Medical School and University of California

Quanticate Sets Out to Becomes a Major New Player in the Global CRO Market

Leading Massachusetts Clinical Research Companies and Organizations Build Bridges to Life Sciences Centers of Innovation in Europe and Latin America

Latest enhancement to its leading electronic data capture (EDC), management and reporting system, Medidata Rave the Dynamic Tabulation Engine.

PharmaPros introduces a new flagship offering, Dataflow Manager, a comprehensive data tracking and integration framework.

The CCI-CPP collaborative team announces the newest release of Country Allocation Planner? (CAP), a Web-based system for global clinical trial planning.??

Octagon announces ViewPoint FUSE 4.2, its Electronic Data Capture solution, which produces submission-compliant output

?Aris Global, a provider of software solutions for the life sciences industry, today announced the general release of agXchange IRT? (inbound receipt and triage) 6.0

New Offering Will Provide Capabilities to Transport, Track and Manage the Status of Clinical Trial Images from within the InForm System, Reducing the Time and Cost Associated with Medical Image Transport

Eli Lilly & Co., a leading developer of pharmaceutical products that help people live longer, healthier and more active lives, took top honors for its support of clinical research education at the 2008 Annual Meeting of the Center for Information and Study on Clinical Research Participation (CISCRP) in June.

Exco InTouch announces the availability of its new technical paper, Electronic Data Capture Using the WOMAC NRS 3.1 Index: a Pilot Study of Cellular Technology in Osteoarthritis.

Clinical Performance Partners, Inc. (CPP) announces the launch of their website (www.clinicalperformancepartners.com).

SMI announced the launch of SMI Clinical Trials, a patient recruitment division designed to accelerate the clinical trial recruitment and enrollment process and reduce per-patient recruitment costs.

Velos, Inc., a resource for clinical trial management information systems for large investigator sites and sponsors, today announced the July 8 launch of Velos eResearch Version 8, bringing customers a host of advanced features and functions on a pure Internet platform.

Quintiles Transnational Corp. names Senior Vice President, Global Marketing and Chief Marketing Officer and Senior Vice President, Communications and Patient Recruitment.

Phase Forward (NASDAQ: PFWD), a provider of data management solutions for clinical trials and drug safety, today announced the Empirica Signal product, a major new release of the company's flagship signal detection and management software formerly known as WebVDME.

CRF Inc., a global provider of electronic Patient Reported Outcomes (ePRO) and wireless data collection solutions for the Life Science Industry, announced the expansion of its world-class Clinical Advisory Board. Comprised of leading clinical and scientific experts, the Clinical Advisory Board will play an important role in providing specialist scientific and therapeutic advice for customers? clinical programs.

Octagon Research Solutions announces CheckPoint software and services to provide over 300 validation checks of electronic clinical study data.

PROMETRIKA, LLC, a contract research organization providing a full range of clinical research services for clients in pharmaceuticals, biologics and medical devices, today announced that Faith Haines Kolb has joined the company as senior director of clinical operations and data management. ??

Novotech, the largest Australian owned contract research organization (CRO) has confirmed its position as the pre-eminent Australian based CRO with the award by leading industry analyst house Frost & Sullivan as the Australian CRO of the Year.

Introducing Applied Clinical Trials' EDC News, a newsletter with news, articles, and information about EDC, IT, and eClinical.

XClinical jointly exhibits with its Japanese CRO partner Asklep at the CDISC Japan Interchange. XClinical has been selected for an ODM training course and for a presentation in the area “Vendor Applications and Tools.”

Octagon Research Solutions’,Chairman and Chief Executive Officer Jim Walker was named as a “Best Chairman” finalist in the 2008 American Business Awards.

Experienced industry veteran joins OmniComm’s executive team to drive technology operations for leading electronic data capture (EDC) company.