News



Information technology improvements support implementation of enhanced adverse event reporting software.

CEO & Chairman Jim Walker of Octagon Research Solutions, Inc., a provider of breakthrough software and services to the life sciences industry, today announced that Octagon will be exhibiting at the Drug Information Association's (DIA) 7th Annual Electronic Submissions Conference.

Nextrials? Prism wins gold in the mobile star awards for health care data capture technology.

Premier Research achieves double-digit growth in functional sourcing for the third straight year.

CTI upgrades to new releases of remote data entry and adverse event reporting products from Oracle

DSA, a global leader in pharmaceutical safety and pharmacovigilance, announced major hardware and software upgrades to support the implementation of the new Empirica Trace adverse event reporting software.

Industry news focusing on the people and organizations who work in the clinical trials profession.

ACR Image Metrix doubles its initial goal for its first fiscal year.

The Foundation for Improving Data Quality (FIDQ) advances the evaluation of clinical trial methodologies that aim to improve the clinical integrity and reliability of clinical data.
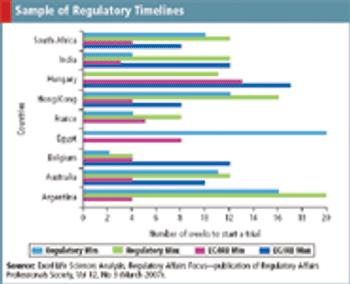
Finding a global regulatory GPS of ethics committee and competent authority approvals.

The European Parliament's Pediatric Regulation has seen its first approval for a centrally authorized drug to be used in children.

PPD, Inc. today announced the Mario Family Foundation has pledged $5 million to the Rutgers University Ernest Mario School of Pharmacy.

ECRFPlus achieves milestone multimillion dollar enterprise agreement with CRO and launches e-CRF+(TM): a CTMS and EDC Web platform with ECG analysis and cardiac safety module.

TAKE Solutions' Life Sciences division announced today the release of PharmaReady Version 4.1 that offers new enhancements to the fully integrated, Web-based suite of products.

Health officials/patient advocates link underrepresentation of minorities in clinical trials with higher death rates.

Medidata Solutions, a provider of clinical trial solutions, today announced the launch of Medidata Developer Central, a Web-based community that supports developers? efforts to integrate their clinical trial solutions with Medidata Rave, an electronic data capture (EDC), clinical data management (CDM), and reporting system.

Clinical Trials & Surveys Corp. (C-TASC), a clinical trials solutions company, announced today that it has been awarded a contract from the National Institutes of Child Health and Human Development (NICHD) to provide clinical trial management services for a study to be conducted by the Division of Epidemiology, Statistics and Prevention Research (DESPR).

Opinions on paediatric investigation plans.

Phase Forward, a provider of data management solutions for clinical trials and drug safety, today congratulated its customer GSK on winning a 2008 Technology Innovation Award from The Wall Street Journal.

Epistem plc, a UK biotechnology and contract research company, will present results from their latest plucked hair biomarker study at the ASCO-NCI-EORTC Meeting on Molecular Markers in Cancer, Hollywood FL, United States on Friday 31st October 2008.

New director appointed to the Office for Human Research Protections (OHRP).

PRA International, a Clinical Research Organization, announced today the appointment of Dr. Jerome Wilson as Senior Medical Director of Late Phase Services.

Rare disease patients, academic researchers, NGOs and industry united their voices in a call to EU governments to take action this Autumn to address the issues facing patients affected by rare diseases in Europe.

Qualia Clinical Services, a division of Holmes Biopharma, selects ALPHADAS, Logos Technologies electronic data capture (EDC) system to streamline and automate clinical trials in its Phase I clinical facility in Omaha, Nebraska.


Qualia Clinical Services, a division of Holmes Biopharma, selects ALPHADAS, Logos Technologies electronic data capture (EDC) system to streamline and automate clinical trials in its Phase I clinical facility in Omaha, Nebraska.

PPD, Inc. has reported its financial and operating results for the third quarter ended September 30, 2008.