
News





















LABS : Quality Image Analysts Save Time and Money CRO/SPONSOR : A Comparison of the Two Types of Sourcing SUBJECT RECRUITMENT : Online vs. Traditional Outreach Also in this issue : The New REMS, UK?s Clinical Trial Regulations Updated, Guidelines for Sponsor-CRO Set to Change, Surveys Reveal Unease with Prospective Observational Research

Central Labs EastBoston, MA September 20-22, 2010 Inventive Early Phase Trial Design Discordance Between BICR Readers Recording Adverse Events

Clinical Trials : A Retrospective Trials in Asia CRO Selection Criteria Ensuring Quality in Clinical Trials Clinical Investigator Survey Interpreting the 1572
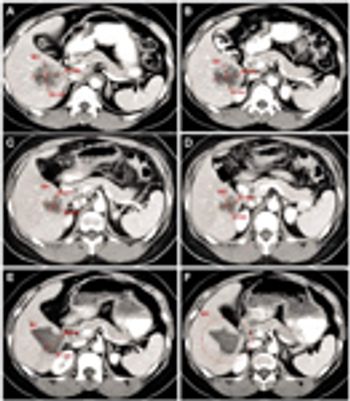
Understanding the causes and implementing processes to mitigate preventable sources of discordance.


Industry news focusing on the people and organizations who work in the clinical trials profession.


Updates on HCV therapies.
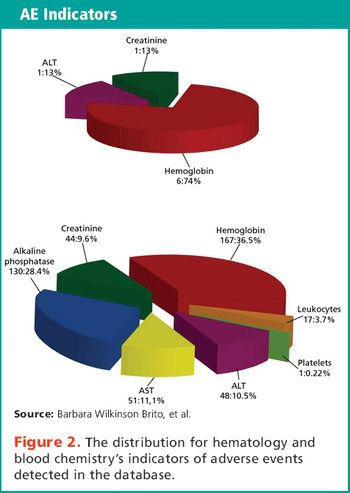
Analysis of hematology and blood chemistry's AEs in sponsor's databases.