
News
Advertisement


Advertisement




Advertisement












SITES : Reducing the Challenges of Study Coordination Workload Measurement Instrument for Cancer Trials RECRUITMENT : African Americans in Clinical Trials Also in this issue : 2011, A Challenge for the Biomedical Research Community?, Joint Manifesto Tries to Address Vicious Cycle, Discontent with IRBs, R&D Success Rate Continues to Fall

Updates on obesity drug development.
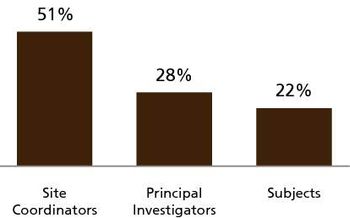
Throughout the entire clinical development, who has the most interaction with prospective and active subjects? Who is primarily responsible for the quality of the source data that ultimately decides the approvability of all biopharmaceutical products?

Industry news focusing on the people and organizations who work in the clinical trials profession.








Advertisement
Advertisement
Trending on Applied Clinical Trials Online
1
Evinova, Bristol Myers Squibb Partner to Apply AI to Global Clinical Development
2
SCOPE Summit 2026: How Payment Practices and Operational Burden Are Changing Site Behavior
3
ACT Brief: Purpose Meets Operational Focus, Acceleration Shifts Upstream, and AI Delivers Near-Term Impact
4
SCOPE Summit 2026: How Sponsors Are Prioritizing AI Adoption Across Clinical Trials
5