
News
Advertisement


Advertisement




Advertisement


Protecting Subjects: The IRBs Next Steps

SITES : Incorporating Standard of Care in Study Budgets Benefits of CTMS at the Investigative Site Also in this issue : FDA Transparency Efforts to Impact Research, Survey Assesses Europe?s Clinical Trials Directive, Tapping into the Potential of Pharmacists, Biosimilars Make Headway in the U.S.

Updates on Osteoporosis including Amgen and Prolia trials.
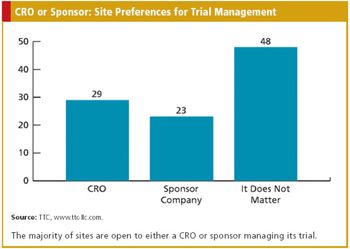
Do Sites Prefer a Sponsor Company or CRO to Run Their Clinical Trial?

Industry news focusing on the people and organizations who work in the clinical trials profession.

















Advertisement
Advertisement
Trending on Applied Clinical Trials Online
1
Thermo Fisher, Datavant Partner to Advance Real-World Data Interoperability Across Clinical Development
2
Addressing Regional Variation in Clinical Trial Risks
3
Evinova, Bristol Myers Squibb Partner to Apply AI to Global Clinical Development
4
Applied Clinical Trials: February 2026 Interactive Digital Edition
5
