
News
Advertisement


Advertisement



Advertisement




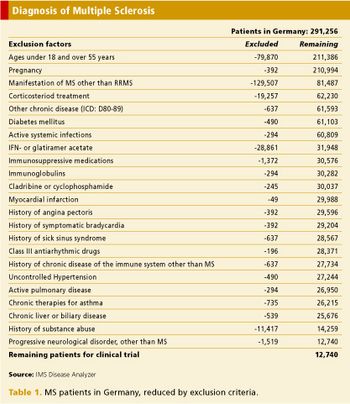
Validating protocols and identifying investigators with secondary data sources.


















eCLINICAL : Global Advantages with CTMS From EDC to ETMS The Upfront Cost Hurdle of EDC eDiaries: Earlier is Better SUBJECT RECRUITMENT : Recruitment Grows on the Internet Also in this issue : FDA Wants Better Science, Flawed EU Directive, BRIDG for Better Models, NICE Gets Bad Rap
Advertisement
Advertisement
Trending on Applied Clinical Trials Online
1
SCOPE Summit 2026 Panel Discussion: Diversity in Clinical Trials—What’s Working, What’s Next
2
SCOPE Summit 2026 Keynote Panel: Is Radical Acceleration in Clinical Research Possible?
3
SCOPE Summit 2026: Reducing Patient Burden Is the Foundation of Wearable Success in Oncology
4
SCOPE Summit 2026: Elevating Patient Experience in Clinical Operations
5