
Wingspan Technology released eTMF 2.1

Industry news focusing on the people and organizations who work in the clinical trials profession.

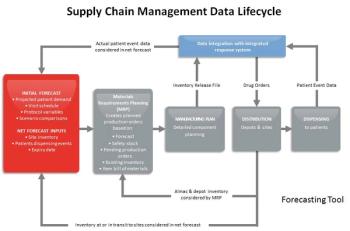
Almac has combined three pre-existing technologies in one support package.

Almac's clinical Supply Chain Management service combines consultancy and supportive technology that helps sponsor companies



The new Clinical Trials Regulation introduces an overhaul of the existing regulation of clinical trials for medicinal products in the EU.

Implementing a "just-in-time" site activation process effectively can significantly speed trial startup.

It is a tragic truism to remark that the merits of the pharma sector are most sharply perceived only at times of deep human suffering.

The health sciences industry received some important and encouraging news from the US Food and Drug Administration in late June...

Last August, Quintiles announced its intention to purchase Novella Clinical, specifically for its focus on small and mid-sized oncology biopharma clients, as well as medical device and diagnostics companies.

As pharmaceutical and biotech companies look to speed development, lower cost, and reduce technical risks associated with new drug development, they are increasing the number and types of collaborative relationships they are forming with other developers.

According to Tufts CSDD R&D Management Report, more than half of all new drugs approved in the United States between 2000 and 2011 were developed by companies that collaborated in one form or another with other entities.

ICR has announced two new courses for December 2014 about the impact of the European Union regulation and essential line management.

Fujitsu Limited and the Alliance for Clinical Research Excellence and Safety announced the formation of a Strategic Alliance

As the biopharmaceutical industry continues to move down an outsourcing path, it is essential that logical sourcing strategies and operational processes exists and align with corporate strategy.

Fujitsu and ACRES have linked up to address challenges facing the development of medicines and health-related research endeavors.

While at the 2014 NYBIO conference, Dr. Sam Waksal from Kadmon had the opportunity to address the audience regarding the state of the biopharmaceutical industry.
Pharmaceutical sponsors conducting clinical trials in the Asia Pacific region have a rare opportunity at hand...

Expansion of Venn's service offering into Data Management & Randomisation

Venn Life Sciences announced the acquisition of Cardinal Systems SAS

IAOCR and the Association of Clinical Research Professionals have announced a new collaborative agreement

Rare disease research offers a lot for people in the world of clinical trials and drug development to think about.

IAOCR and the Association of Clinical Research Professionals (ACRP) are pleased to announce a new collaborative agreement.

Pressure to shorten study start-up timelines puts clinical supply management in the crosshairs.
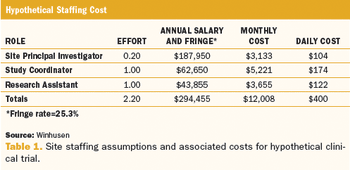
Method designed to reduce the time gap between protocol approval and recruitment is examined.

New phase of innovation initiative emphasizes focus on research networks, adaptive trials, and personalized medicine, among other key areas.

eGSP-driven approach focuses on overcoming operational challenges through data integration.

Consumer groups, research organizations, and Congress are pressing for more inclusive clinical research.