
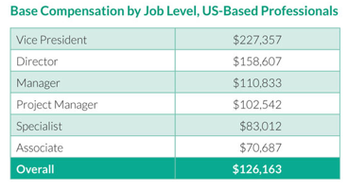
The advent of Accountable Care Organizations is causing providers to rethink their legacy financial models as they seek to effectively quantify and understand the clinical or financial experience of their patients.

The advent of Accountable Care Organizations is causing providers to rethink their legacy financial models as they seek to effectively quantify and understand the clinical or financial experience of their patients.
RAPS has released the results of its biennial survey of the global healthcare regulatory profession.

DIA held its 2014 annual meeting in San Diego and attracted thousands of attendees from around the globe.

Children and young people should routinely be offered the opportunity to participate in and to benefit from medical research.

As the new European Parliament assembles for the start of its five-year term on July 1, many familiar faces will be missing.

Amid all the heated discussions of transparency and the insistence on ever wider disclosure, an intriguing contrast is provided by a document signed by health ministers on June 20.

May be time to review current restrictive guidance in UK and elsewhere

Prisoners are being unfairly excluded from taking part in potentially beneficial clinical research, on the grounds that it would be too difficult and expensive to include them...
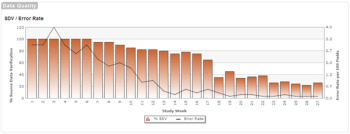
This is the third post in a three-part series on quality implications of RBM.

We got the chance to speak with Nancy Dreyer, chief of scientific affairs and senior VP at Quintiles Outcome about the current tide for comparative effectiveness research

Debuting at BIO, Covance MarketPlace Connects Emerging Biotech with Potential Partners Early in the Development Process

Covance announced the introduction of Covance MarketPlace, a new solution that enables Covance?s emerging biotechnology and established pharmaceutical clients to find and forge new partnerships in a secure forum.

Progress on new research and treatments for dementia has been "achingly slow," noted Dennis Gillings, PhD, Executive Chairman of Quintiles.


Benchmark Research is the latest participant in the Association of Clinical Research Professionals' Certification Supporting Partner Program.

Alliance will rapidly expand sponsors and CROs access to advanced predictive, implementation, and operations support technologies

ACRP and the European Centre for Clinical Research Training announce the introduction of the ECCRT/ACRP STAR Program.

Privacy Analytics, Inc. announced the availability of a new solution designed to enable pharmaceutical companies to share clinical trials data while protecting the privacy of the individual patients.

agCapture 3.2 Delivers on the Promise of Real Unification of Data Management Processes

Provider of patient-driven eData Systems will run data collection system on Dell Venue 11 Pro tablets with Windows 8.1.

PHT Corporation announced that it has selected Dell Venue 11 Pro tablets featuring Windows 8.1

New Solution Responsibly Safeguards Data, Accelerates Sharing and Fosters Greater Transparency with Patients and Researchers

Historical information and analytical foundations for RBM are important, but, in my view, RBM is about proactively managing each individual trial to quality and efficiency goals.

The Certified Clinical Research Associate designation is now formally recognized by TransCelerate Biopharma, Inc. as evidence of Good Clinical Practice training.

CenterWatch and TrialReach have joined forces to offer patients access to the largest set of clinical trials resources in the industry.

TrialReach and CenterWatch Partner to Offer Patients Access to the Largest Set of Resources in the Clinical Trials Industry

'Me&MyCOPD' mHealth program utilizes interactive mobile phone and internet-based health tools to improve condition management for patients suffering from COPD, as well as their caregivers and healthcare providers.

SAS Life Science Analytics Framework helps pharmaceutical, biotechnology, and medical device manufacturers effortlessly gather, standardize, and analyze research data from start to finish

eClinical Leader adds Risk-Based Monitoring Technology to Platform

SAS Analytics lets researchers securely access clinical trial data from multiple sponsor companies