
The Belgian health ministry has decreed that Belgian lung-cancer patients can receive and be reimbursed for immunotherapy treatments.

The Belgian health ministry has decreed that Belgian lung-cancer patients can receive and be reimbursed for immunotherapy treatments.

Policy makers, sponsors and regulators are taking steps to promote alternative study formats and methods that go beyond randomized clinical trials.

The challenges of data quality are a constant factor in clinical trials, especially that of traditional paper source documenting. Joyce Smith of The Medical Research Network speaks about the study’s site perspective on trial data quality.

Our series on patient centricity continues with a patient perspective on her interpretation of this subject. Shelly Hoover, an ALS patient, shares her views with Moe Alsumidaie.

Issues of counterfeit drugs being introduced into a supply chain have been a concern in comparative clinical trials. Terry Walsh of GSK speaks about the addressing this issue and ensuring that comparator drug supplies are more readily available for comparative trials.

The EU’s national health organizations are contemplating to abandon their highly-prized autonomy, and forge common positions and buying strategies for accessing expensive new drugs.

The Senate confirms Scott Gottlieb as FDA commissioner. User fee reauthorizations, a hiring freeze, and the opioid epidemic are a few of the issues awaiting the agency's new leader.

As negotiations between the UK and the remaining 27 members of the European Union commence in the next month or so, questions of trade, IP protection and of free movement will emerge.

The recent partnership between Continuum Clinical and Lyft has introduced the convenience of transportation into the hands of patients. Nariman Nasser, VP of Site Optimization at Continuum Clinical, elaborates on this partnership.

At the end of April, European Immunization Week will take place celebrating the achievements of immunization in healthcare. However, contemporary sentiments of skepticism threaten to dampen the event.
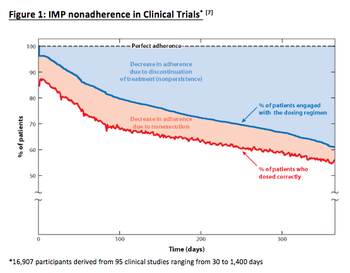
As clinical trials remain costly and continue to increase, the promise of novel initiatives gives hope that trial duration and cost impact will drop. However, biopharma continues to overlook one element that affects study timelines: patient non-adherence.

The adoption of electronic health records by hospitals and providers has increased engagement in Health Information Technology (HealthIT) activities. Nora Belcher of the Texas eHealth Alliance speaks on how HealthIT will change the pharma landscape for drug development.

The adoption of electronic health records by hospitals and providers has increased engagement in Health Information Technology (HealthIT) activities. Nora Belcher of the Texas eHealth Alliance speaks on how HealthIT will change the pharma landscape for drug development.

In continuing our series on patient centricity, we are adding the digital perspective from inside of a biopharmaceutical enterprise. In this interview with Jeremy Sohn, VP, Global Head of Digital Business Development & Licensing at Novartis, we will delve into how biopharmaceutical enterprises are incorporating digital strategy into patient centric study execution, and elaborate on some of the cultural challenges of adopting novel study methods.

An international panel of industry experts has concluded that randomized, controlled clinical trials provide the fastest and most reliable way to identify the risks and benefits of treatment candidates for infectious disease outbreaks.

Jeremy Sohn, VP, Global Head of Digital Business Development & Licensing at Novartis, delves into how biopharma is incorporating digital strategy into the execution of patient centric studies.

At his confirmation hearing before the Senate Health, Education, Labor and Pensions Committee, Scott Gottlieb addressed concerns about the FDA’s future.

A late-March volley of support from the European Union came in the form of a prize awarded to the new European Reference Networks, designed to connect patients with rare diseases to experts across Europe.

Wherever the EMA winds up after Brexit, the reality for anyone working on drug development is that the strains and constraints of their job are not going to get any easier.

Is the much anticipated Europe action plan on personalized medicines, unveiled recently by an EU-sponsored international consortium, worth paying a close eye on?

Meghan McKenzie, Associate Director and Sr. Clinical Program Lead at Genentech, elaborates on her experiences in the field of patient centricity from the perspective of the sponsor.

John Reites, formerly of Quintiles, and current Chief Product Officer at THREAD Research, discusses how mHealth is incorporated in clinical trial patient centricity.

The last piece in our three-part series on improving efficiencies and sponsor/CRO collaboration through advanced CTMS, reveals a way in which CROs can best their competition in the marketplace.

Centralized monitoring is the core of RBM and helps study teams decide on on-site monitoring activities and site intervention. Thus, the right mix of KRIs and Central Statistical Monitoring Reports is crucial for trial success.

Clinical trials have changed significantly in the past decade with increasingly large and complex global studies. Multi-center, multinational trials are common, with complicated treatment protocols, large staffs, and huge data sets muddling the clinical trial processes. With years of development time and millions in R&D invested in a new treatment before it reaches the trial stage, there is incredible pressure on investigators to deliver quality data.

The second in our three-part series, Improving efficiencies and sponsor/CRO collaboration through advanced CTMS, explores how technology can foster better collaboration.

A recent eConsent survey reinforces the notion that this tech innovation is the next clinical trial solution the industry will adopt.

The first in this 3-part series, Improving efficiencies and sponsor/CRO collaboration through advanced CTMS, reveals the quickest way that sponsors and CROs can save money and thus keep trials affordable and efficient.

President Trump’s nomination of Scott Gottlieb as the next FDA Commissioner has given pharma a reason for calm, though a lot will be on his plate.

A recently released study regarding off-label prescribing in Europe has caused confusion as it lacked clarity on how the issue of how to govern off-label use going forward.