
Centralized monitoring is the core of RBM and helps study teams decide on on-site monitoring activities and site intervention. Thus, the right mix of KRIs and Central Statistical Monitoring Reports is crucial for trial success.

Centralized monitoring is the core of RBM and helps study teams decide on on-site monitoring activities and site intervention. Thus, the right mix of KRIs and Central Statistical Monitoring Reports is crucial for trial success.

A technology-driven approach to oversight of vendors and sites can provide sponsors with timely and proactive solutions toward minimizing risks.
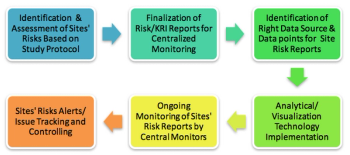
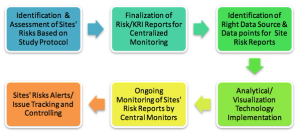
Centralized monitoring is a suitable way in which sites can identify and control investigational risks while improving performance. This new approach can not only help sponsors to monitor site performance, but also facilitate proper oversight resulting in good ROI.

Investigational sites’ readiness to support new monitoring initiatives such as risk-based monitoring and centralized monitoring will dictate their success. Here are key aspects that sponsors and CROs should establish with investigational sites while implementing these new initiatives.

Identifying Key Risk Indicators (KRIs) is an important step in successfully applying risk-based monitoring initiatives to a clinical trial. These factors, within risk management, assist by defining risk areas in order to measure and monitor them centrally throughout the trial.

With the introduction centralized monitoring in a risk-based monitoring (RBM) approach, the central monitor is emerging as a key and important role.

Using the five steps to a risk management plan from other industries--Identify, Assess, Plan, Track and Control--to inform a RBM approach in clinical trials.

The successful implementation of centralized monitoring requires effective planning, process restructuring, cross-functional expertise alignment, and the right technology in place.

Published: October 1st 2015 | Updated:

Published: March 4th 2016 | Updated:

Published: March 23rd 2016 | Updated:

Published: May 24th 2016 | Updated:

Published: August 25th 2016 | Updated:
Published: November 3rd 2016 | Updated: