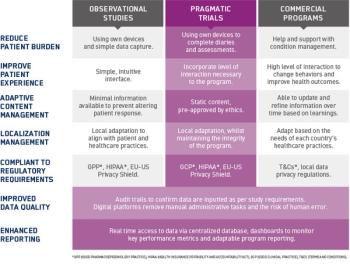
In this three-part blog series, we look at how electronic platforms can support and enhance data capture in several types of RWD programs: observational studies, pragmatic trials, and commercial programs.

In this three-part blog series, we look at how electronic platforms can support and enhance data capture in several types of RWD programs: observational studies, pragmatic trials, and commercial programs.
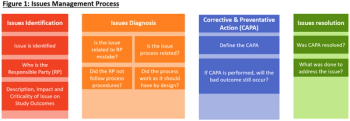
In this article, we will define a process on how to identify, diagnose, correct, and resolve issues in a QMS issues management repository.

Joe Pollarine, Head of GxP Systems Strategy Director at Janssen, recently spoke about CRO oversight models and will expand on these models in this interview.
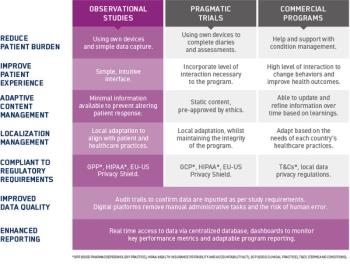
In this three-part blog series, we look at how electronic platforms can support and enhance data capture in several types of RWD programs: observational studies, pragmatic trials, and commercial programs.

At the October Metrics in Patient Centered Drug Development conference hosted by DIA, many of these key constituents gathered to talk about how to implement and measure the impact of patient input.

The operational subject of how the centralized authorization procedure is going to work when the UK is no longer part of the European Union is being discussed.

The biopharmaceutical industry must establish customized approaches to managing Quality Management Systems within their clinical trials processes.

Jill Wechsler talks about biosimilars and the requirement of clinical trials in her recent blog.

In this blog by Peter O'Donnell, the EU provides a report on the progress its made within the pediatric medicines regulation.

As the size, complexity, duration, cost, and globalization of clinical trials has grown, pharmaceutical and biotech companies have moved to outsource clinical activities to CROs to achieve a wide range of objectives.

As concerns grow about the slow progress of advanced therapies, drug developers are pushing for action from European authorities.

Tensions mount across the European pharmaceutical scene over the fate of the EMA with just weeks before the final decision is going to be made on where it will be moved to.

FDA advisory committee made up of patients comes together to improve clinical trials and product development through better study design and greater participation.

Most physicians and many specialists have limited understanding of clinical trial data and research findings presented in prescription drug promotional materials for professional audiences.

The Duke Margolis Center for Health Policy (DMCHP) recently collaborated with the FDA to release an mHealth action plan entitled "Mobilizing mHealth Innovation for Real-World Evidence Generation."
This article will dive into the details of FDA�’s movement in mHealth, analyze FDA’s approach, and assess how this movement impacts the use of mHealth in clinical trial settings.

New safety review of epilespy treatment puts spotlight on issues such as communication between regulators and prescribers.

There is a critical need to rethink standards of evidence and of the reliability of information used to make regulatory decisions. According to the FDA, this involves placing greater reliance on data from sources outside traditional clinical studies and because of these new tools for collecting the data, the FDA needs to adapt as well.

The EMA isn't mincing its words about the challenge it faces with the relocation that will be forced on it by Brexit. It has been stated that "the future of public health in Europe is at stake" and this decision will make or break it.

Gaps in the good manufacturing practice controls on medicines for clinical trials are targeted in new rules from the EU.

Hugo Stephenson MD examines his longing for the past with the advances of today's technology in this blog focused around sites.
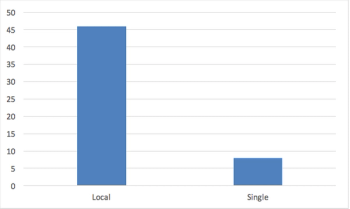
Since the emergence of the independent IRB sector, sponsors have found that regulatory and ethical review by a single IRB yields important benefits with respect to efficiency, high quality, and consistency in human research protections.

Austin Speier, VP of Emerging Technologies at Precision for Medicine, recently spoke about mHealth regulatory pathways at PanAgora’s Mobile Innovations Summit, and will elaborate further in this Q&A interview.

Jeff Lee had the opportunity to be a moderator at the Mobile in Clinical Trials event where he sat down with mobile experts within the pharmaceutical industry. The session allowed for new observations in regards to the value that these mobile technologies have within clinical trials.

With the ever changing regulatory landscape, outsourcing non-core activities is a flexible approach to managing resources and remaining compliant with NCO requirements for the entire generic portfolio.

George Clinical's Managing Director discusses the concern biopharma companies have with CFDA's slow drug and medical device approvals.

This article outlines the EMA's response from a previous blog by Peter O'Donnell regarding the potential of Milan, Italy as the EMA relocation.

What cannot have escaped the attention of any impartial observer is that Milan is one of the leading contenders to host the EMA and that Tajani and Rasi are both Italian. The EMA relocation is a hot topic without any bias towards Milan.

The National Institutes of Health (NIH) focuses on developing strategies that will encourage further research to support safe and effective therapies for pregnant and lactating women.

This article discusses best practices and benefits of keeping a trial top of mind at global sites, and measuring some of the qualitative and quantitative impacts it can have on a trial.