
In this interview, Jim Streeter, Global Vice President of Life Sciences Product Strategy at Oracle, will elaborate further on Oracle’s strategy with mHealth in clinical trials.

In this interview, Jim Streeter, Global Vice President of Life Sciences Product Strategy at Oracle, will elaborate further on Oracle’s strategy with mHealth in clinical trials.

The British government has at last set out its ambitions for cooperation on medicines post-Brexit.

In this interview, Linda Rawlings, VP of Neurodegenerative Development at Synteract, elaborates on addressing the challenges of neurodegenerative disease clinical trials.

Given the rapid changes in the communication landscape brought about by participative Internet use and social media, it is important to develop a better understanding of these technologies and their impact on health communication.

Patients stand to lose the most when sponsors hold too tightly to drugs doomed to fail. This wastes precious time that patients don’t have and squanders valuable resources.

FDA is testing various strategies to streamline research and regulatory oversight by looking to novel clinical trial designs to advance new treatments.

Although Biogen's Alzheimer's drug BAN2401 addresses buildup of amyloid plaques, investors and scientists look to reevaluate the plaque theory and search for a glimmer of hope in breakthrough treatments.

A continued struggle for healthcare and healthcare innovation to be taken seriously by the EU.

The late-June announcement that Ireland is joining the Beneluxa Initiative on Pharmaceutical Policy might suggest renewed vigour for the drive to equip national governments with more clout in their pricing negotiations with international drug firms.

The general assumption that the Policy 0070 data releases were public data releases can be challenged since there is a mechanism to enforce the ToU, and this mechanism will continue to exist after the Agency moves to the Netherlands.

How much does the EU really care about health, and about the infrastructure that is a precondition to successful healthcare and healthcare innovation?

The right-to-try gale is now blowing at full strength in the US and is likely to prove a straw in the wind that could become a haystack.
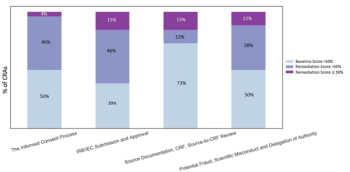
In this article, we will demonstrate a methodology that resulted in improving CRA performance.

Liquid biopsy, typically non-invasive blood-based tests for circulating tumor DNA and circulating tumor cells, has been long-hyped as a potential game-changer for cancer treatment.

In this article, we will discuss trends and challenges with medical monitoring clinical analytics, analyze the alignment of existing medical monitoring tools and technologies with ICH-E6 (R2) addendum guidelines, and discuss trends in medical monitoring insourcing and outsourcing models.

To manage the growing scope and complexity of global disclosure regulations and trial transparency initiatives, companies are advised to create a cross-functional clinical transparency committee.

During an audit, the FDA investigates six areas to determine whether a site is in compliance with federal drug accountability regulations-can sponsors answer them?

Editor-in-Chief Lisa Henderson speak about therapeutic needs, rare diseases in children, and the SCORR Marketing survey on innovative or flexible trial designs.

Peter O’Donnell explores the convergence of policy and science in new vaccine R&D pursuits in Europe.

Peter O'Donnell discusses the EU's bid to improve the HTA and weighs in on a report made by Soledad Cabezón's.
John Reites, THREAD’s Chief Product Officer, will discuss eDROs and the FocalView App in this interview.

Right-to-Try bill sent to White House for President’s signature after passage by Congress.

Artificial intelligence is a "new weapon" in healthcare research.
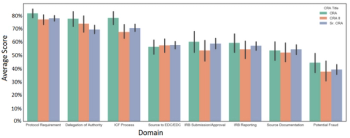
A global CRO’s data gathered using an objective monitoring simulation administered by CRA Assessments, LLC reveals that CRAs are consistently underperforming regardless of the level of experience or training.

With the rise in gene therapies entering clinical trials, it is important to address the operational challenges associated with these types of trials.

The biopharmaceutical industry continues to explore how mHealth can change clinical trials, as the discussion continued at Hanson Wade’s mHealth for Clinical Trials EU Summit in London.

On Europe Day, the agency itself is right now keener than ever to assess its worth and the value of its services to Europe and to Europeans.

In this interview, Munther Baara, Head of New Clinical Paradigms at Pfizer, will discuss his perspectives on how blockchain may impact clinical trials.

FDA officials make efforts to better manage the increasing amount of applications for new drugs that treat disease in innovative ways and include new kinds of clinical research, starting with a plan to restructure the Office of New Drugs (OND) in the Center for Drug Evaluation and Research (CDER).

Provenance is at the heart of the discussion when it comes to the equation of privacy and provenance.