
As the clinical trials industry starts to incorporate mHealth initiatives into their trials, study teams now have to determine the extent of their ICF consent forms during study deployment, and how to cover mHealth activities within these forms.

As the clinical trials industry starts to incorporate mHealth initiatives into their trials, study teams now have to determine the extent of their ICF consent forms during study deployment, and how to cover mHealth activities within these forms.

The release of the ICH E6 R2 guidance, which outlines a new approach to clinical trials operations, will have a major impact on the industry, writes Patrick Hughes.

In this interview, we speak to Dr. Joanne Waldstreicher, Chief Medical Officer of J&J’s Office of the Chief Medical Officer (OCMO), to discuss its data sharing program via the Yale University Open Data Access (YODA) Project.

Of the 28 European Union member states, nine did not make an offer to host the European Medicines Agency (EMA) by the July 31 deadline-and one of them is very sorely missed, writes Peter O'Donnell.

How can manufacturers ensure their clinical trial methods are evolving along with their product portfolio? It starts by taking a patient-focused approach to trial design, writes Susan Weidner.

The European Health Data Network (EHDN) initiative kicks off-a challenging and resource-intensive effort designed to harmonize data analysis and conversion amid the push toward outcomes-focused healthcare in Europe.

With the rise in deaths, injuries, and treatment costs related to the abuse and misuse of opioid painkillers, the biomedical research community is seeking more effective and informative methods for testing and evaluating new pain medicines. Jill Wechsler reports.

Jill Wechsler discusses the importance of clinical trial participants and the challenges FDA faces in expanding patient engagement.

Peter O'Donnell discusses Brexit challenges and how the life sciences industry is affected.

In this article, MedCision executive discusses cell therapies.

This Q&A discusses how the movement in making studies more patient-centric continues, and Pfizer is now implementing its own mHealth study in Lupus.

Peter O'Donnell discusses the interest expressed by members states to host the EMA.

The FDA ruling that exempts biosimilar makers from waiting an extra six months after approval to distribute a new product should help overcome delays in future biosimilar sales, writes Jill Wechsler.
In this article, we will analyze themes from asthma patient conversations via HealthUnlocked, an online healthcare social network, and compare these themes to asthma clinical trial endpoints from large pharma studies.

Peter O’Donnell discusses the argument on whether or not European researchers should be allowed to continue using non-human primates in their research.

Rob DiCicco, VP of Clinical Innovation and Digital Platforms at GSK, will discuss the TransCelerate protocol template initiative in this interview.

Peter O'Donnell discusses Estonia taking over the rotating presidency of the Council of the European Union.
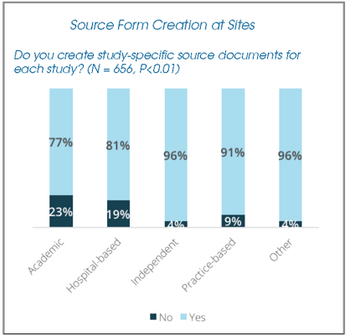
Tanya Bridges and Donna Benson, from two non-affiliated study sites, discuss the burdens of using paper source forms, and their impact on resources and trial execution at study sites.

4G Clinical head of marketing, Amy Ripston interviews Catherine Hall, director of operational excellence, at Sunovion to discuss how IRT bridges the gap between clinical and supply, and how interactive response technologies (IRT) can be leveraged to enable innovative trial designs.

We interviewed Pfizer exec, Dr. Jonathan Rowe, about how they are leveraging predictive models to manage study risk and quality.

As clinical trials have become more complex and costly, traditional paper-based data management systems have increasingly proved impractical and ineffective. Cloud-based technologies fit the bill, and industry professionals have begun to recognize this reality and reap the benefits at an ever-increasing rate.

CDISC executive talks about the creation of their cloud-based platform to free standards from PDF documents.

We discuss the pilot siteless models in clinical trial patient centricity with Gabriel Vargas, an executive at Amgen.

Several projects in works illustrate the importance of those unsung heroes in Europe's R&D engine room.

We explore the remote trial and hybrid models in clinical trial patient centricity with Hassan Kadhim, an executive at Boehringer Ingelheim.

In order to successfully support the globalization of clinical research, sponsors and CROs must empower global research sites and treat them as valued business partners.

The Clinical Endpoints Adjudication Group and Ethical Clinical designed and implemented an industry survey to evaluate factors that drive the use of endpoint adjudication in clinical trials.

The European generic drug industry homed in on the opportunity to “help the EU and member states develop effective policies that support access to medicines for patients.”

Nearly every EU member country has put in a bid to host the EMA once the UK leaves the European Union in 2019. The conditions and criteria for doing so are now becoming clear.

How pragmatic clinical trials increase the robustness of real world studies at a fraction of the cost of classical randomized controlled clinical trials.