
Peter O'Donnell writes about the results of a public opinion survey, the latest the European drug industry's attempts to fight back against what it sees as a climate of misunderstanding.

Peter O'Donnell writes about the results of a public opinion survey, the latest the European drug industry's attempts to fight back against what it sees as a climate of misunderstanding.

Tony Fiorino, Chief Medical Officer of electroCore, discusses challenges he has faced with device studies, and will elaborate on his experiences about the differences between device and drug trials.

A trial’s success depends on enrolling the planned number of patients in the shortest possible time frame. Such thinking can insight a limited focus on obtaining and analyzing all investigator site enrollment data - as if that would solve all problems pertaining to site enrollment performance, writes Gen Li, President of Phesi.

FDA draft guidance for modernizing the approach to clinical trial design for oncology drugs and biologics look to make clinical trials more efficient while maintaining patient safety.

C.K. Wang, MD, senior medical director at COTA Inc., discusses the impact of RWD and RWE in clinical oncology.

Bill Tobia, CEO of Clinstruct, Ahmed Bouzid, CEO, Witlingo, and Brielle Nickoloff, Lead Product Marketing, Witlingo, discuss how voice will change the clinical research paradigm.

Penelope Manasco, CEO of MANA UBM, disputes recommendations made by the original authors she finds to be problematic and worthy of further discussion.

Peter O'Donnell writes of the European Commission's recently published reports suggesting that some of the gaps were being filled in registries in the field of rare diseases.

The World Health Organization tries to find a balance with its Road Map for Access on how to best approach research and pricing in Europe.

Kyle Hogan, Director, eClinical Solutions, Clinical Ink, writes how however promising ePRO may be - its benefits may not be enough to overcome concerns that take an significant period of investment before generating business, patient benefits, and financial returns.

The case for evaluating drugs with real-world digital health data.

New money is not going to solve problems when it comes to registries in Europe, Peter O'Donnell writes.

Moe Alsumidaie sits down with Ping Yeh, CEO and co-founder of StemoniX, to discuss how MicroOrgans will transform preclinical and clinical development in challenging areas like Alzheimer’s.

CEO of MANA UBM, Penelope K. Manasco, explores the different approaches to determine what the right 'easy button' is to push to achieve effective clinical trial conduct and oversight.

John Ebeid, Senior VP of Randstad Life Sciences writes that though International Clinical Trials Day is a time to celebrate the medical researchers who work to make new discoveries for the good of public health - retaining these employees remains a challenge for the entire industry, especially for today's CROs.

Where pharmaceutical interventions for Alzheimer's falls short, nursing care facilities are relying heavily on non-traditional medicines and physical and mental exercise.

As elections near, politicians in Europe make promises to prioritize the fight against cancer.
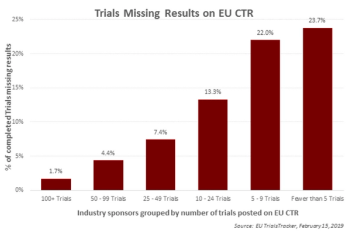
Transparency in clinical trials doesn't have to be difficult but requires attention writes Thomas Wicks, Chief Strategy Officer for TrialScope. Smaller organizations tend to lag in their commitments to clinical data sharing and be non-compliant with regulations, but the trend is shifting.

Peter O'Donnell writes about Manfred Weber's announcement of what he called "his European Master Plan against Cancer" – with what he termed "a guarantee" for European citizens, taking political lobbying over health to a new level in Europe

Dr. Mark Smith, Chief Medical Officer of VistaGen Therapeutics, sits down with Moe Alsumidaie to discuss the challenges of modern psychiatric clinical trial design and implementation and their neuroactive pherine, PH48B.

Nonadherence in clinical trials plays a significant role in influencing the quality of data, trial results and, subsequently trial cost and duration. It may stem from unintentional drivers, such as forgetfulness, poor organizational skills, protocol regimen complexity, or experiencing an Adverse Event.
After launching as a free online resource for country-specific, clinical research regulatory information in 2014, ClinRegs has grown to over 68,000 users from 157 countries.

A new CDER “knowledge management” approach will see companies submit applications that can be transmitted to experts from multiple disciplines able to assess applications for new drugs and biologics in a timely and efficient manner.

Vice President of Global Technology and Product Management, Chris Dailey, and Enterprise Architect at Cenduit LLC, Chris Driver, encourage all sponsors and CROs to take a strategic view of how to make an eClinical ecosystem function at its highest level.

After taking a long awaited step forward in a rare treatment, the impatience of European patient organizations are understandable given the slow emergence of advanced medicines. Peter O'Donnell writes there is good news for patients - just not quite as good as they may have thought.

A statement from Europe’s drug industry and Europe’s medical societies calls for a “bold vision” to ensure proper funding and coordination for translational research.

CROs should be more mindful of their smaller emerging biopharma clients and pay more attention to handling their needs.

CEO of Greenphire, Jim Murphy, shares his takeaways from 2019 SCRS European Site Solutions Summit and illustrates how to further position clinical research stakeholders for success.

Discussions on collaborative health technology assessment have been so intense over the last year that it might be thought that Brussels is the hub for HTA action. Peter O'Donnell writes how there is plenty going on elsewhere in Europe.

Karen Hill and Heather Fitzpatrick Medlin discuss the recent advancements and challenges in cardiovascular and metabolic studies, as well as strategies to address current obstacles and advance the industry further.