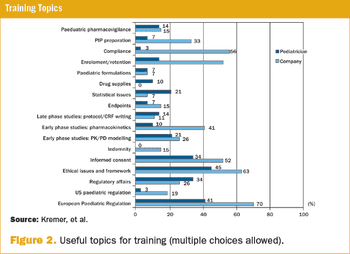
A survey on the perception of European pediatricians and industry/CROs

A survey on the perception of European pediatricians and industry/CROs

A discussion of summary findings from the CISCRP 2013 Perceptions & Insights Study
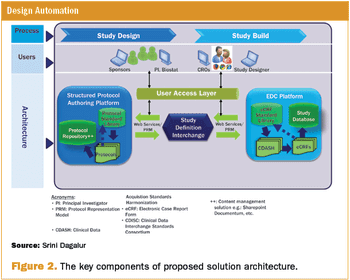
Incorporating CDISC-based libraries to store study components for study design and building eCRF pages.

Clinical research processes need to be simplified before new technology can be properly utilized.
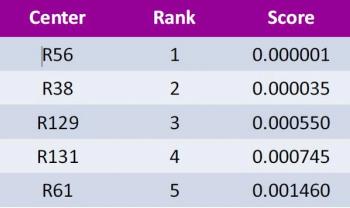
In a phase III trial in vascular disease, over 4,500 patients were randomized across 160 sites.

Physician participation in and patient access to cancer treatment clinical trials (CCTs) are key measures for delivery of quality cancer care.
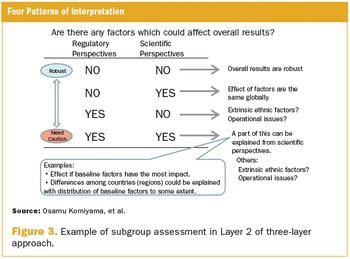
Planning for ethnic sensitivity from a scientific and regulatory perspective.

Bring Your Own Device (BYOD) makes sense economically and socially and is the future of clinical trials.

Adaptive trial designs have the potential to transform success rates, but require new operating strategies and practices.

Multinational pharmaceutical companies are under intense financial and competitive pressures to make the drug development process more effective and efficient, and to expand into a greater number of international markets.

The 505(b)(2) new drug application (NDA) provides a potentially streamlined path for sponsors who have developed improvements to drug products that have previously received FDA approval.

Despite the growing need for pediatric-approved medications, clinical trial enrollment for pediatrics remains challenging.

Expect the FDA to be looking at a new metric in the drug approval process: driving safety.

Central labs promote scientifically objective results, through independence of action on a contractual basis with the sponsor.

Transfer of knowledge between pre-clinical and clinical research is necessary to deliver effective medicines to patients.

Work is needed if true efficiencies are to be gained in clinical trial performance.
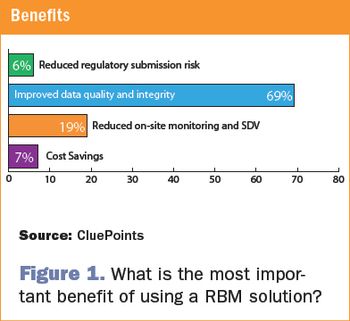
Central statistical monitoring can improve RBM solutions by efficiently detecting errors, sloppiness, tampering, and fraud.
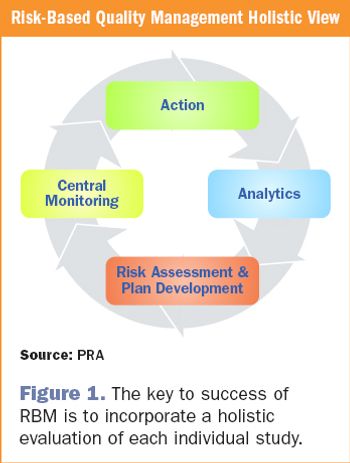
The reality of risk-based monitoring: history and successful implementation for late phase research.

The eClinical Forum Risk Based Monitoring Taskforce offers some best practices for ensuring clinical data quality.

The use of RBM may be the only way forward as long as the development of medicines relies on clinical testing.

Risk-based monitoring ushers in the move from the traditional experimental design.

Addressing patient dropout with an arsenal of tools that leverages both technology and communication.
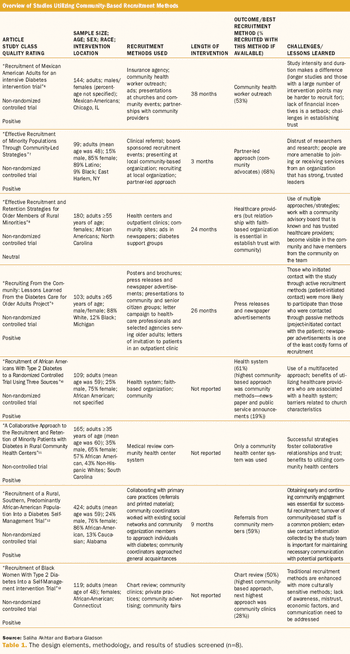
Community-based recruitment engages multiple stakeholders in a collaborative approach.
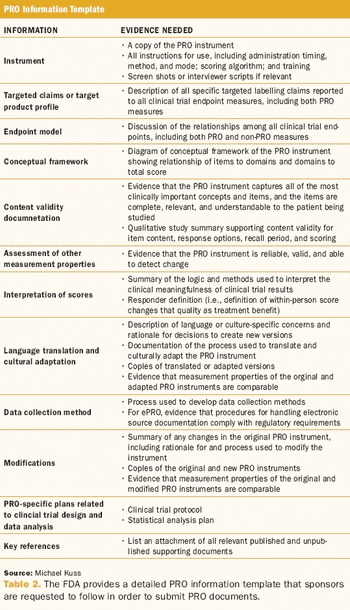
PRO measures enrich the evaluation of treatment effectiveness and help sponsors differentiate products.

Changing demographics and evolving standards of care bring additional challenges when creating protocol designs.