
Darcy Flora, PhD, chief research officer of GRYT Health, a digital oncology company, discusses her career, the company, and drug development.

Darcy Flora, PhD, chief research officer of GRYT Health, a digital oncology company, discusses her career, the company, and drug development.

Meeting patients at the point of care can increase trust and engagement.

Some actions to consider for your next trial or submission.

Elderly patients achieved up to 98% compliance at 18 months on Phase III study.

Now that the industry has been forced to make room for new digital processes, there should be no turning back.

This article sets out a vision of adopting convenient alternative blood sampling approaches that places the needs of the patient at the center and enables active monitoring, before the onset of clinical events, leading to improved healthcare.

Nicole Richie, Global Head Health Equity and Population Science at Genentech/Roche, discusses a renewed strategy in big pharma to incorporate diversity inclusion in clinical trials.

Physicians share their experiences integrating research into practice.

Study designs, coupled with difficulties finding, enrolling and retaining trial participants, further intensify operational challenges and prevent new treatments from getting to market.
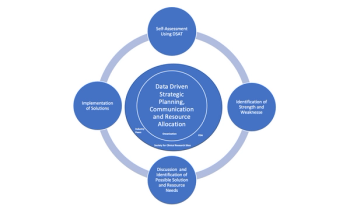
While it's important to note that there are areas where the industry can improve, there are unique opportunities of improvement for specific types of sites and the number of studies conducted there.

Andaman7 and Afrisda, Inc. sign partnership agreement to support digital health implementation in Africa.


Centralized medical imaging providers must challenge their current processes to improve patient diversity.

How COVID-19 blazed a new trail for greater patient inclusion.

Affirming and advancing patient engagement and digital transformation.

The role of data science, how it can help us understand patient behavior, and why digital adherence solutions are the future.

Sparked by the pandemic, patients are now leveraging remote technology to take control of their healthcare.

Michele Russell-Einhorn, Chief Compliance Officer at Advarra discusses safety, speed, and ethics in COVID-19 vaccine trials.

Building authentic trust lays at the heart of creating more patient-centric trials.

Understanding the role of social determinants on clinical trial participation and trust.

Co-conveners of the newly formed Decentralized Trials & Research Alliance, Amir Kalali, MD and Craig Lipset discuss the alliance as well as how COVID-19 has impacted the industry.

Hybrid studies, patient-centric tools, and collaborations create big changes for clinical trials and stakeholders during COVID-19.

EVP and General Manager of Hū, April Lewis, discusses why the industry has seen a decline in trial participation and what Hū is doing to combat it.

Eliminating barriers to engage underrepresented populations.

Randomized controlled trials establish strict inclusion and exclusion criteria in an effort to optimize the cohort of trial participants.