
News








eCLINICAL : Paperless IRB - Streamlined Efficiency Translating Data into Meaningful Metrics STATISTICS : Review of Categorical Data Analysis LABS : Industry Perspective of Good Clinical Laboratory Practice Also in this issue : Pharmaceutical R&D Funding, Europe 2020 Strategy, Protocol Amendments, Predicting Adverse Events

Oncology & Clinical Trials in the 21st Century CAM Use in Asia-Pacific Personalized Medicine Cancer Biomedical Informatics Grid How CROs Help Oncology Sponsors
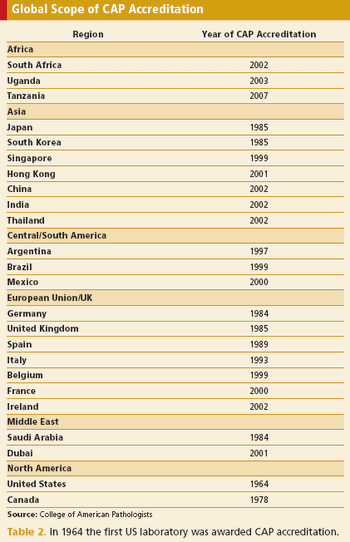
Application of good clinical laboratory practice reduces risks to subject safety and data integrity.
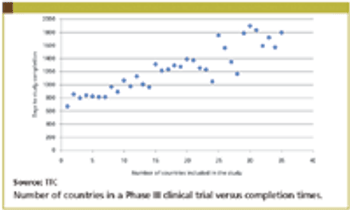
Improved study completion times represents one of the most critical areas for clinical development organizations. Previous TTC research has demonstrated that paying sites more on a cost per patient basis does not improve study completions times.

Industry news focusing on the people and organizations who work in the clinical trials profession.

Going paperless can help an IRB quickly collaborate, share documents, and review protocols.

A new study shows that the cost of implementing protocol amendments should be weighed carefully.

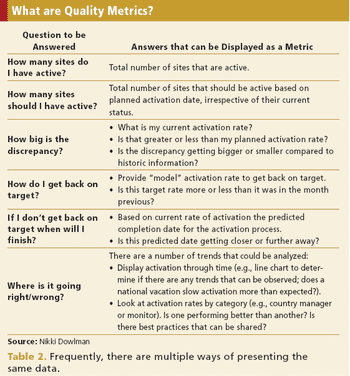
How to define information requirements to enhance the understanding of a clinical trial.

New portable eClinical suite Timaeus HotSpot allows for quicker and more secure data transfer.

A review of different statistical methods for data types and models used during research.

Europe's financial problems have resulted in new blackslists of drugs among other challenges.

Cover

DIA is increasing its capacity in both live and web-based educational events.








