
News



TRIAL DESIGN : Enrollment is More Than a Numbers Game REGULATORY : Late Phase PK Collection Also in this issue : Protocol Design and Study Management, Effects of Europe?s Economic Turbulence, Ready for Big Data?, Outcomes Assessments

Medidata Solutions
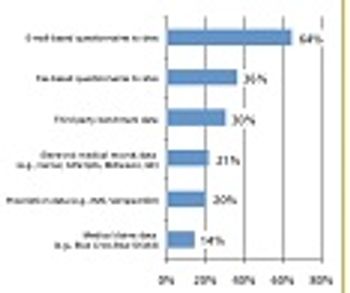
Industry Standards Research

The best relationships between sponsors and contractors are those that are well defined from the outset.

PPD has begun training its Clinical Research Associates in a virtual classroom.

Industry news focusing on the people and organizations who work in the clinical trials profession.

Five key pieces to a lasting and successful sponsor/CRO relationship.

PAREXEL's Neal Mantick discusses the strategic advantage of the transition from clinical research to commercial.

Vendors have developed systems for massive databases, but are we data ready?
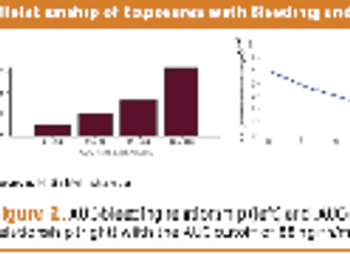
Collecting PK data in late phase trials led to alleviating additional trials, drug approval, and better dosing.

ACT December 2011 BPA Statement

Over the past year and a half we have been monitoring the trend towards adoption of risk-based monitoring across the industry, and in particular the move towards reduced source document verification (SDV).















